Triamcinolone acetonide acetat and preparation method thereof
A technology of cream and curmi, applied in the field of new curmi cream and its preparation, can solve the problems of uneven distribution of main ingredients, easy instability of cream, loss of effectiveness of cream, etc., to achieve industrial application, The preparation method is simple and easy to control, and the effect of anti-allergy is rapid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
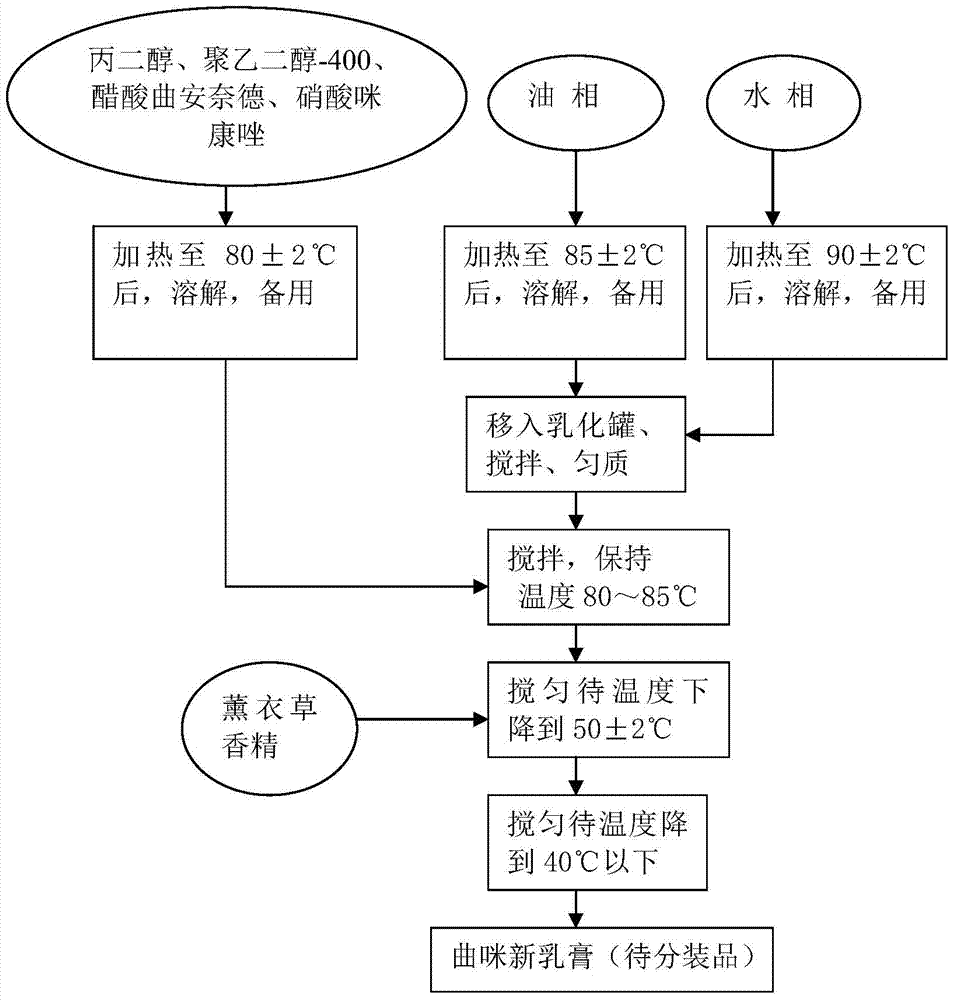
[0034] A kind of curmixin emulsifiable paste is obtained by the preparation method of the following steps, and its preparation process schematic diagram is as follows: figure 1 Shown:
[0035] (1) Prepare each batch of raw materials according to the following prescription, the total weight of each batch is 400kg: triamcinolone acetonide acetate 0.4kg, miconazole nitrate 4kg, neomycin sulfate 1.2 billion units, propylene glycol 19.2kg, polyethylene glycol-40028.8 kg, C16-18 alcohol 23kg, glyceryl monostearate 15.5kg, white petrolatum 3.0kg, stearic acid 3.8kg, isopropyl myristate 19.2kg, Pingpingjia-O 6.1kg, lavender essence 0.4kg, purified water balance;
[0036] (2) Preparation of main drug solution: propylene glycol, polyethylene glycol-400, triamcinolone acetonide acetate, and miconazole nitrate of the above prescription amount are put into an insulated mixing tank, heated in a water bath, and constantly stirred to dissolve completely, Keep warm at 80±2°C for standby;
...
Embodiment 2
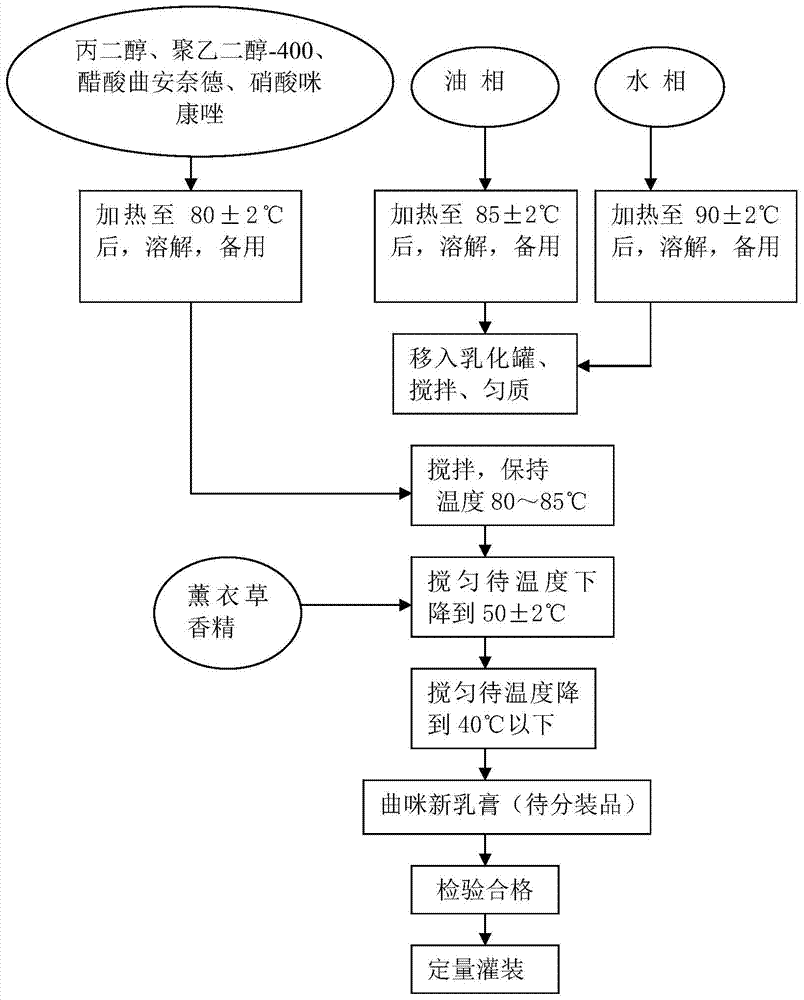
[0045] A kind of curmixin emulsifiable paste is obtained by the preparation method of the following steps, and its preparation process schematic diagram is as follows: figure 2 Shown:
[0046] (1) Prepare each batch of raw materials according to the following prescription, the total weight of each batch is 400kg: triamcinolone acetonide acetate 0.4kg, miconazole nitrate 4kg, neomycin sulfate 1.2 billion units, propylene glycol 19.2kg, polyethylene glycol-40028.8 kg, C16-18 alcohol 23kg, glyceryl monostearate 15.5kg, white petrolatum 3.0kg, stearic acid 3.8kg, isopropyl myristate 19.2kg, Pingpingjia-O 6.1kg, lavender essence 0.4kg, purified water balance;
[0047] (2) Preparation of the main drug solution: put propylene glycol, polyethylene glycol-400, triamcinolone acetonide, and miconazole nitrate in the above-mentioned prescription amount into an insulated stirring tank, heat in a water bath, and the temperature of the water bath is 80-85°C. Stir continuously to dissolve ...
Embodiment 3
[0057] A kind of curmixin emulsifiable paste is obtained by the preparation method of the following steps, and its preparation process schematic diagram is as follows: figure 2 Shown:
[0058] (1) Prepare each batch of raw materials according to the following prescription, the total weight of each batch is 400kg: triamcinolone acetonide acetate 0.4kg, miconazole nitrate 4kg, neomycin sulfate 1.2 billion units, propylene glycol 19.2kg, polyethylene glycol-40028.8 kg, C16-18 alcohol 23kg, glyceryl monostearate 15.5kg, white petrolatum 3.0kg, stearic acid 3.8kg, isopropyl myristate 19.2kg, Pingpingjia-O 6.1kg, lavender essence 0.4kg, purified water balance;
[0059] (2) Preparation of the main drug solution: put propylene glycol, polyethylene glycol-400, triamcinolone acetonide, and miconazole nitrate in the above-mentioned prescription amount into an insulated stirring tank, heat in a water bath, and the temperature of the water bath is 80-85°C. Stir continuously to dissolve ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com