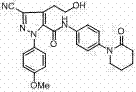
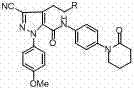
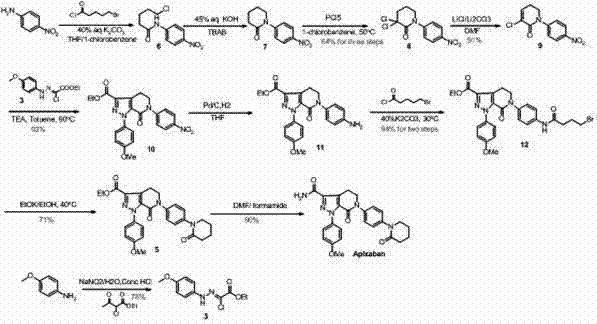
Method for preparing antithrombotic drug apixaban
An antithrombotic drug, apixaban technology, applied in the direction of organic chemistry, etc., can solve the problems that products are difficult to meet API standards, difficult to large-scale production, and limited industrial production, and achieve large-scale production, easy large-scale production, etc. Simple and convenient production and post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] About the preparation steps of compound A:
[0047]
[0048] Step 1: Dissolve p-methoxyaniline in tetrafluoroboric acid and water, cool down to -10°- -5° with an ice-salt bath, slowly add a solution of sodium nitrite dissolved in water, and keep the temperature at 0°- -5 °, after the dropwise addition, the reaction continued to stir for 20-50 minutes, preferably the stirring time was 30-40 minutes, then filtered, the filter cake was washed with cooled ether, and the filter cake was vacuum-dried at room temperature to obtain a gray solid product p-methoxy Aniline diazonium tetrafluoroborate, wherein the molar ratio of methoxyaniline, tetrafluoroboric acid, sodium nitrite and water is 1.0:1.0:1.0:10-1.0:5.0:5.0:50, which includes The numerical range of any subinterval of , non-limiting examples: 1.0: 1.5: 1.5: 15-1.0: 4.5: 4.5: 45, 1.0: 2.0: 2.0: 20 -1.0:4.0:4.0:40, 1.0:3.0:3.0:30-1.0:3.5:3.5:35, the preferred molar ratio is 1.0:1.5:1.5:30, Yield: 90-95%.
[0049] ...
Embodiment 2
[0052] About the preparation steps of compound B
[0053]
[0054] Dissolving 5,6-dihydro]pyran-2-one in an aprotic organic solvent, wherein the aprotic organic solvent includes one or more of toluene, dichloromethane, dichloroethane, and chloroform , preferably dichloromethane, keep the temperature at 25°-30°, slowly add a solution of bromine dissolved in dichloromethane dropwise, and stir the reaction for 2-5 hours, preferably 4-5 hours. After the reaction, the reaction system was cooled to 0°-5°, stirred for 0.5-1 hour, the system was diluted with water, the organic phase was separated, the aqueous phase was extracted with dichloromethane, the combined organic phase was washed with saturated brine, and dried over sodium sulfate , concentrated and dried to obtain a colorless liquid product B, Among them, the molar ratio of 5,6-dihydro-pyran-2-one, liquid bromine and dichloromethane is 1.0:1.0:5.0-1.0:3.0:50, and this range includes the numerical range of any subrange the...
Embodiment 3
[0056] Preparation steps of compound Ⅰ
[0057]
[0058] Compound A and Compound B are dissolved in a non-polar organic solvent, wherein the non-polar organic solvent can be one or more of toluene, dioxane, tetrahydrofuran, dichloromethane, dichloroethane, and chloroform, Preferred is toluene, adds alkali, and alkali can be one or more in organic bases such as triethylamine, pyridine, also can be one or more in inorganic bases such as sodium carbonate, sodium bicarbonate, potassium carbonate, potassium bicarbonate. One, preferably triethylamine, warming up to 20°-100°, preferably 80°-100°, stirring for 1-10 hours, preferably 8-10 hours, then reducing the reaction system to 20°-30°, dilute the filtrate with water, separate layers, wash the organic phase with saturated brine, dry over sodium sulfate, and concentrate to obtain a residue, stir and beat the residue in ethyl acetate, filter, and dry in vacuo to obtain a light yellow solid product Ⅰ , wherein, the molar ratio of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com