Application of quercitrin in preparation of human gamma delta T cell proliferator
A technology of cell proliferation, quercetin, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
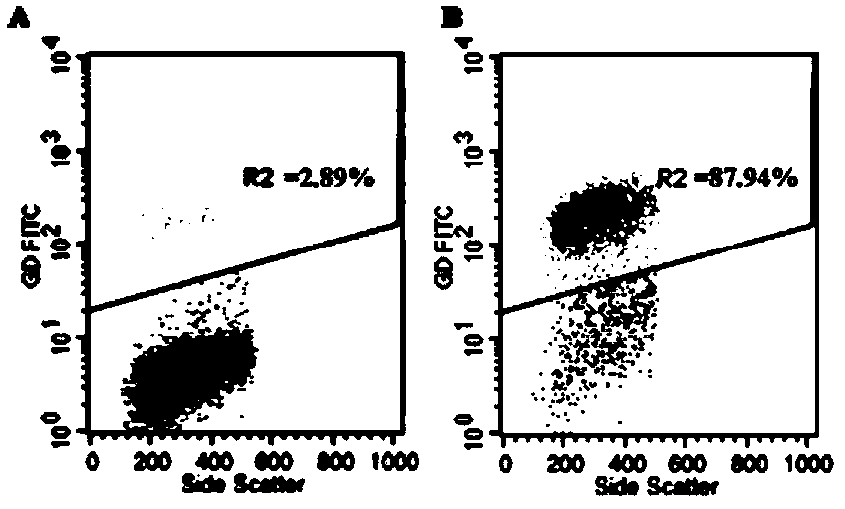
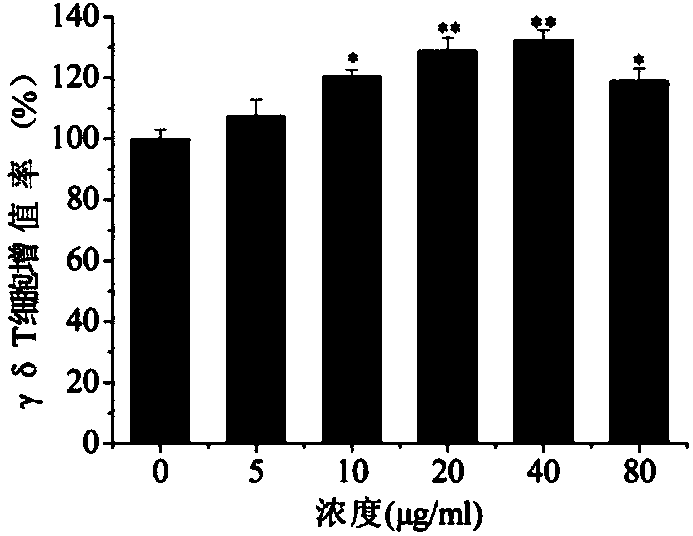
[0015] Example 1: Effect of quercitrin on the proliferation of γδT cells
[0016] 1 Materials and methods
[0017] 1.1 Experimental materials
[0018] FITC-labeled anti-γδTCR, PE-labeled anti-perforin (anti-perforin), PE-labeled anti-granzyme B (anti-granzyme B), Fix&Perm membrane breaker were purchased from ebioscience; recombinant human interleukin-2 (rhIL-2), isopentenylpyrophosphate (IPP) were purchased from Xiamen Tebao Biological Engineering Co., Ltd.; RPMI-1640 medium, calf serum and trypsin were purchased from Gibco; lymphocyte separation fluid was purchased from Institute of Hematology, Chinese Academy of Sciences; CCK-8 kit, BCIP / NBT alkaline phosphatase chromogenic kit, and Western and IP cell lysate kits were purchased from Biyuntian Biotechnology Co., Ltd. Human AB serum was purchased from Xuzhou Blood Bank. Phosphoric acid solution was analytically pure, and water was redistilled water.
[0019] Quercitrin, from the Polygonum capitatum Buch.-Ham.ex D.Don in t...
Embodiment 2
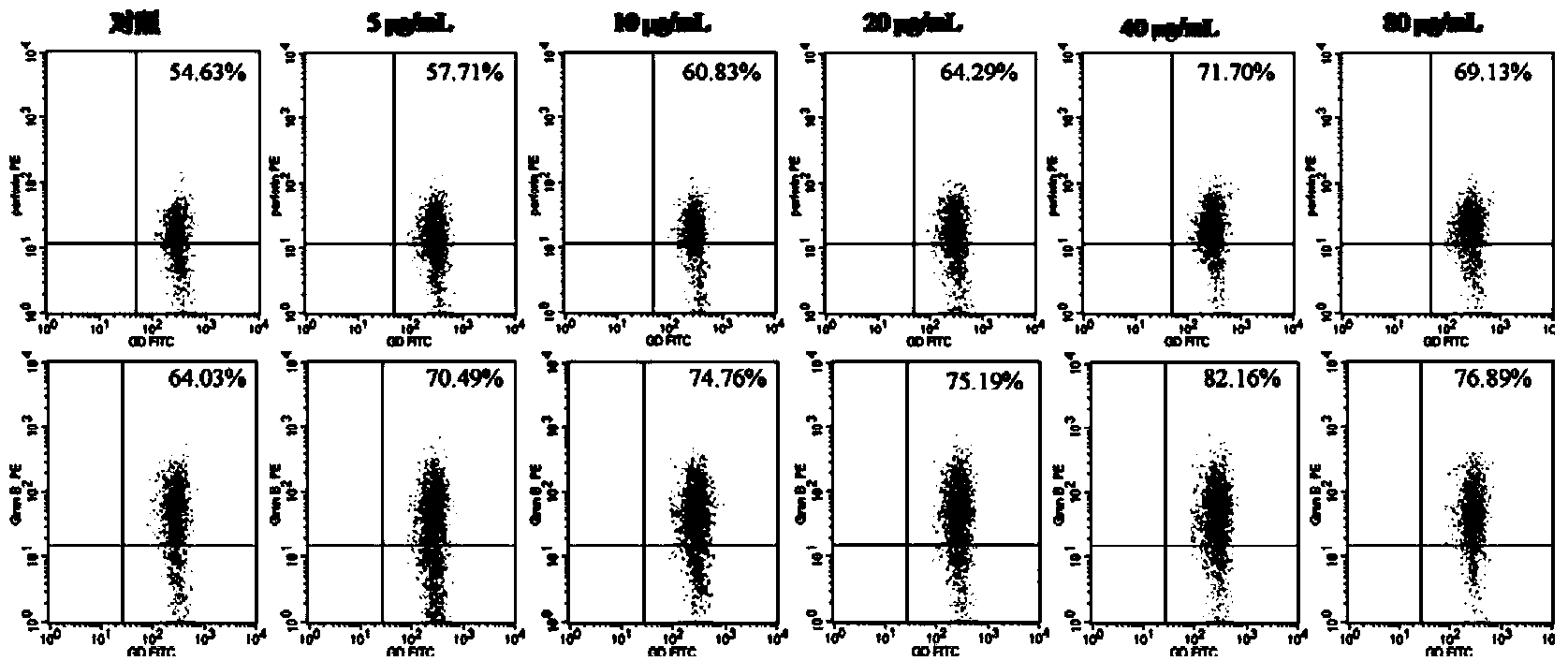
[0031] Example 2: Effect of quercitrin on the expression of perforin and granzyme B on γδT cells
[0032] 1 Materials and methods
[0033] Among them, 1.1 Experimental materials; 1.2 γδT cell culture and identification; 1.3 Statistical processing are the same as in Example 1.
[0034] 1.4 Detection of expression of perforin and granzyme B on γδT cells by flow cytometry
[0035] The successfully cultured γδT cells were made into a cell suspension with a concentration of 1.0×105 / ml, inoculated in a 6-well culture plate, 3ml per well, and then added with final concentrations of 0μg / ml, 5μg / ml, 10μg / ml, and 20μg / ml, 40μg / ml, 80μg / ml quercitrin, each group had 3 replicate wells. At 37°C, 5% CO 2 Cultivate in the incubator for 48h. The cells were collected and washed twice with phosphate buffered saline (PBS), and 20 μl of anti-γδTCR-FITC was added to each tube, and incubated in the dark for 30 min. Add 100 μl of fixative and incubate at room temperature in the dark for 15 min...
Embodiment 3
[0038] Example 3: Effect of quercitrin on Bcl-2, p-ERK1 / 2 and p-Akt protein expression in γδT cells
[0039] 1 Materials and methods
[0040] Among them, 1.1 Experimental materials; 1.2 γδT cell culture and identification; 1.3 Statistical processing are the same as in Example 1.
[0041] 1.4 Western blot detection of Bcl-2, p-ERK1 / 2 and p-Akt protein expression
[0042] The successfully cultured γδT cells were prepared at a concentration of 1.0×10 5 / ml cell suspension, inoculated in 6-well culture plate, 3ml per well, then added quercetin with final concentrations of 0, 5, 10, 20, 40, 80 μg / ml respectively, at 37°C, 5% CO 2 Cultivate in the incubator for 48h. Cells were collected and washed once with PBS, fully lysed and extracted protein with 100 μL of Western and IP cell lysate, the protein concentration of the sample was measured by BCA method, separated by 10% SDS-PAGE, and then the protein was transferred to PVDF membrane, 5% delipidated The milk powder was blocked f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com