Calcium borate silicate biological material as well as preparation and application thereof
A biomaterial, calcium borosilicate technology, applied in dental preparations, dental prostheses, compression molding cups, etc., to achieve the effect of easy operation, low price, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
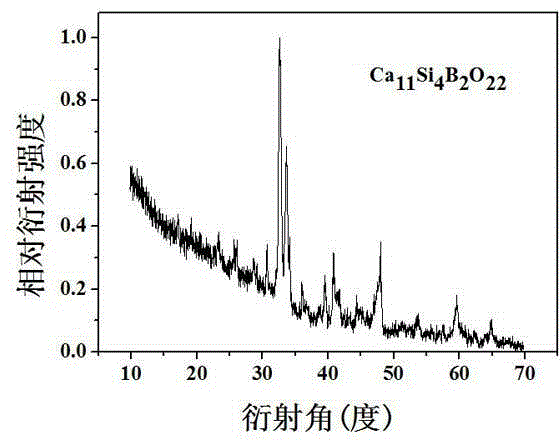
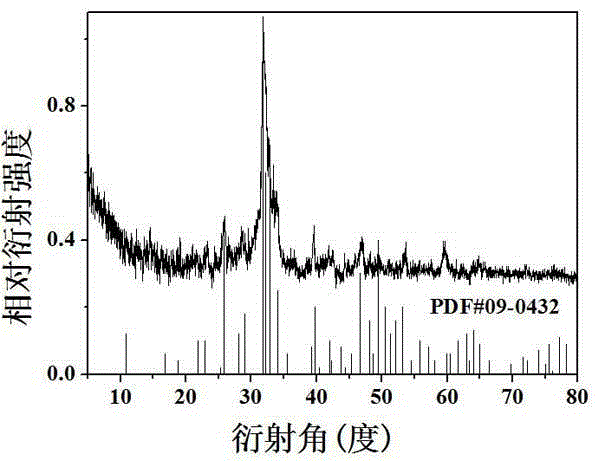
[0041] Embodiment 1: Preparation of Ca 11 Si 4 B 2 o 22
[0042] According to the chemical formula Ca 11 Si 4 B 2 o 22 , respectively weighed calcium carbonate CaCO 3 : 2.75 g, silicon dioxide SiO 2 : 0.609 g, boric acid H 3 BO 3 : 0.3092 g, after grinding and mixing in an agate mortar, select the air atmosphere for the first calcination, the temperature is 350 ℃, the calcination time is 3 hours, then cool to room temperature, take out the sample; the raw material calcined for the first time is fully Mix and grind evenly, then calcinate again in the air atmosphere, the temperature is 850 ° C, the calcining time is 9 hours, then cool to room temperature, take out the sample; finally it is fully ground again and calcined in the air in the muffle furnace, the calcining temperature is 1450 ℃, the calcination time is 15 hours, and the powdered Ca 11 Si 4 B 2 o 22 . The resulting powdered Ca 11 Si 4 B 2 o 22 Soak in simulated body fluid (SBF) for mineralization, ...
Embodiment 2
[0050] Embodiment 2: preparation Ca 11 Si 4 B 2 o 22
[0051] According to the chemical formula Ca 11 Si 4 B 2 o 22 , respectively weigh calcium oxide CaO: 2.0563 grams, silicon dioxide SiO 2 : 0.812 g, boron trioxide B 2 o 3 : 0.2321 g, after grinding and mixing in an agate mortar, select the air atmosphere for the first calcination, the temperature is 400 ° C, the calcination time is 4 hours, then cool to room temperature, take out the sample; the raw material for the first calcination is fully Mix and grind evenly, then calcinate again in the air atmosphere, the temperature is 900°C, the calcining time is 8 hours, then cool to room temperature, take out the sample; finally it is fully ground again and placed in the muffle furnace for air calcining, the calcining temperature is 1400°C, The calcination time is 14 hours, and the powdered Ca 11 Si 4 B 2 o 22 . The resulting powdered Ca 11 Si 4 B 2 o 22 Soak in simulated body fluid (SBF) for mineralization, ob...
Embodiment 3
[0053] Embodiment 3: preparation Ca 11 Si 4 B 2 o 22
[0054] According to the chemical formula Ca 11 Si 4 B 2 o 22 , weigh calcium hydroxide Ca(OH) 2 : 2.7167 g, silicon dioxide SiO 2 : 0.812 g, boric acid H 3 BO 3 : 0.4122 g, after grinding and mixing in an agate mortar, select the air atmosphere for the first calcination, the temperature is 450 ℃, the calcination time is 5 hours, then cool to room temperature, take out the sample; the raw material calcined for the first time is fully Mix and grind evenly, then calcinate again in the air atmosphere, the temperature is 800°C, the calcining time is 7 hours, then cool to room temperature, take out the sample; finally it is fully ground again and placed in the muffle furnace for air calcining, the calcining temperature is 1350°C, The calcination time is 13 hours, and the powdered Ca 11 Si 4 B 2 o 22 . The resulting powdered Ca 11 Si 4 B 2 o 22 Soak in simulated body fluid (SBF) for mineralization, observe the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com