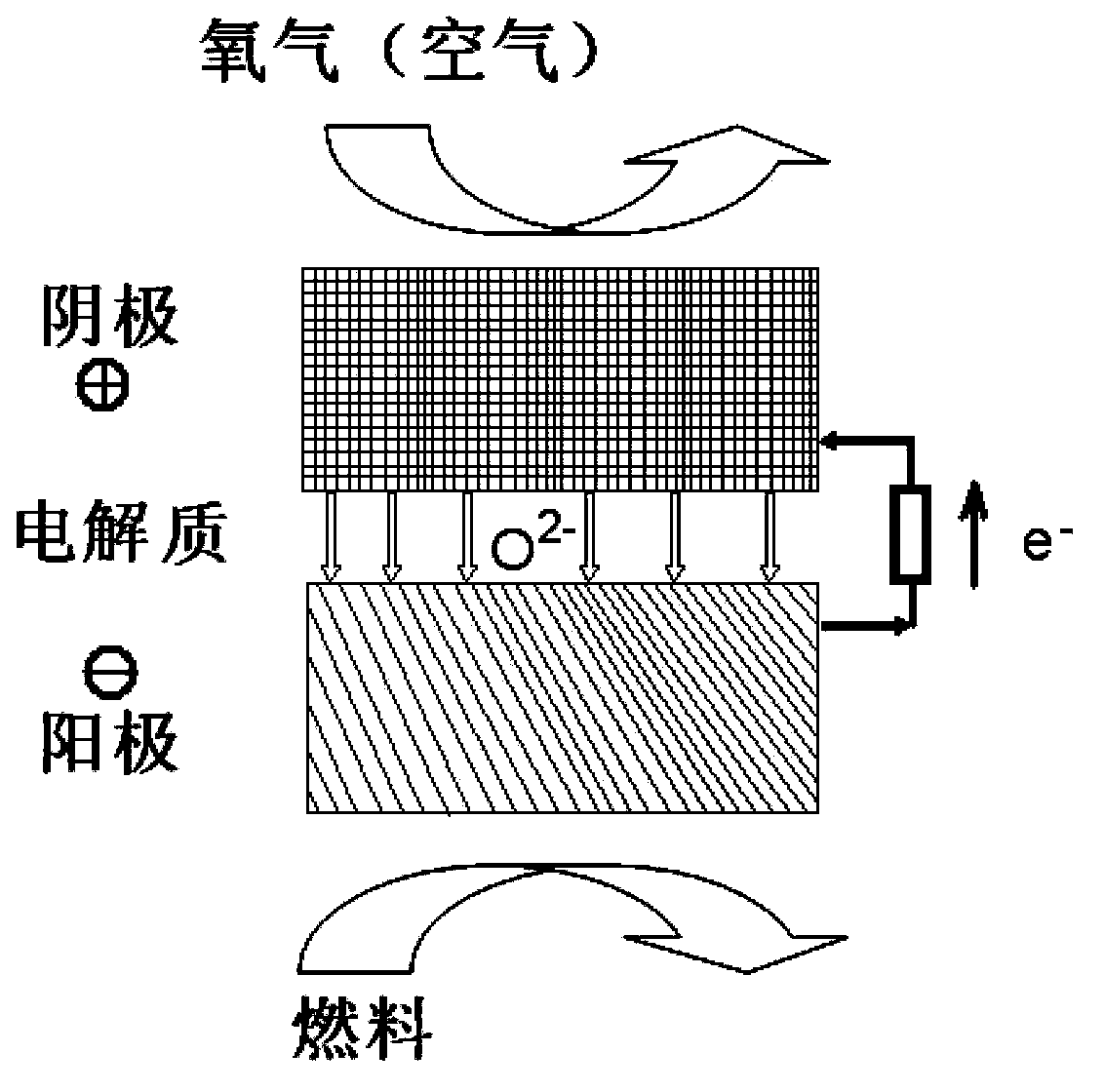
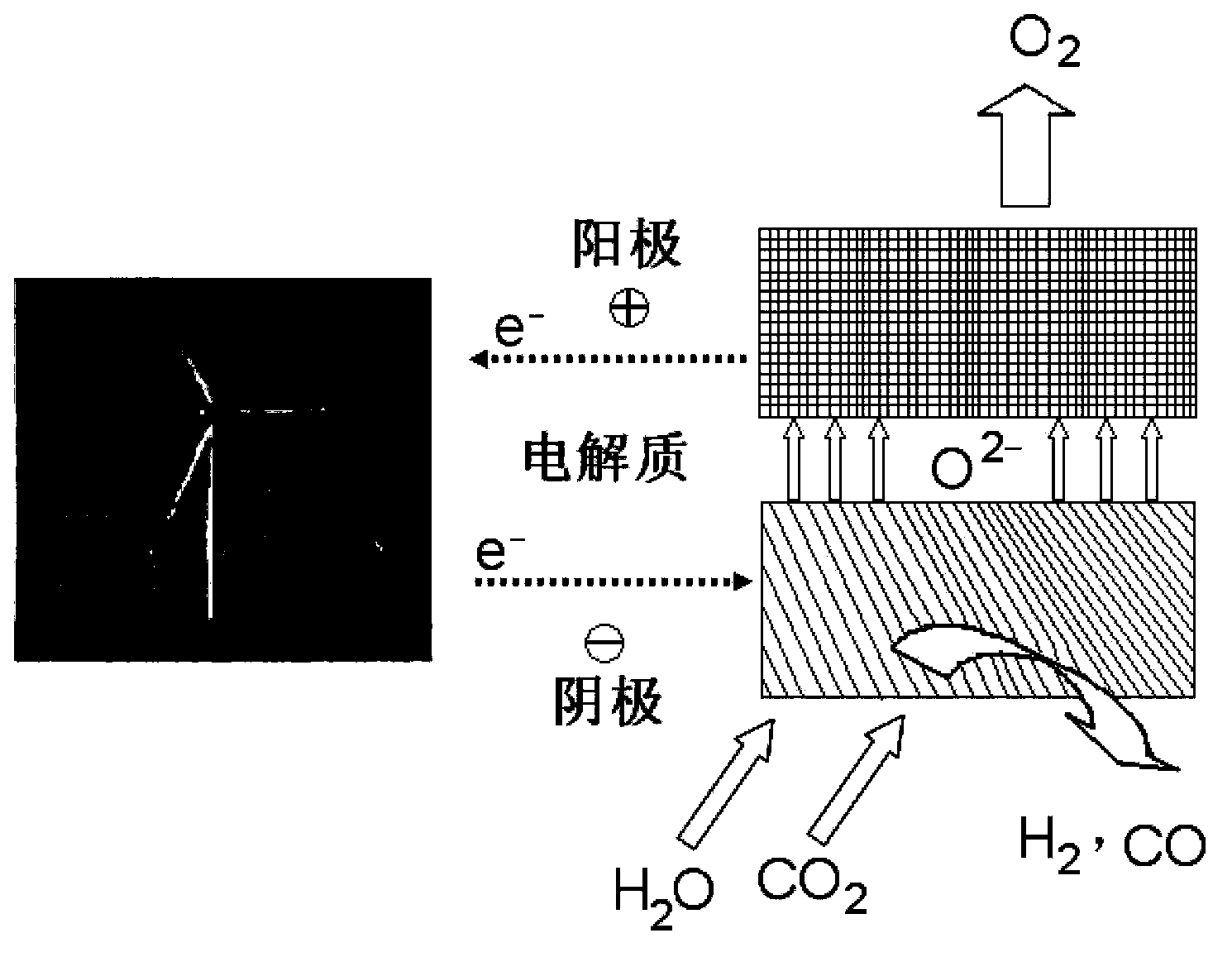
Composite material containing perovskite structure oxide, preparation method and application thereof
A perovskite-type, composite material technology, applied in structural parts, fuel cells, electrochemical generators, etc., can solve problems such as low performance, poor oxygen reduction performance, and concentration polarization of porous structure design cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] This example is used to illustrate the perovskite structure oxide Sr 0.95 Ce 0.05 CoO 3-δ and its preparation method.
[0112] Wherein, the present embodiment adopts sol-gel method to prepare Sr 0.95 Ce 0.05 CoO 3-δ , the specific steps include:
[0113] (1) 2g of analytically pure grade strontium nitrate (Sr(NO 3 ) 2 ), and stoichiometric cobalt nitrate (Co(NO 3 ) 2 ·6H 2 O) and cerium nitrate (Ce(NO 3 ) 3 ·6H 2 O) dissolved in 110 ml of deionized water to prepare a solution;
[0114] (2) Add 8 ml of ethylene glycol and 2.86 g of citric acid to the above solution respectively, wherein the molar ratios of ethylene glycol and citric acid to the total amount of metal ions are about 7.2 and about 1.5 respectively, and stir on a hot plate at 80°C for 10 hour, a brown gel was obtained;
[0115] (3) Put the prepared brown gel into an oven and dry it at 250°C to obtain a black-gray precursor;
[0116] (4) Grind and press the prepared precursor into tablets, sin...
Embodiment 2
[0120] This example is used to illustrate the perovskite structure oxide Sr 0.9 Ce 0.1 CoO 3-δ and its preparation method.
[0121] Wherein, the present embodiment adopts solid state reaction method to prepare Sr 0.9 Ce 0.1 CoO 3-δ , the specific steps include:
[0122] (1) Add 1g of analytically pure SrO 2 , and the stoichiometric CeO 2 and Co 3 o 4 Grind in an agate mortar to mix well;
[0123] (2) Press the powder obtained by grinding in step (1) into tablets, sinter in air at 1100°C, repeat the grinding, pressing and sintering operations until no impurity phase is detected, and the total sintering time is about 72 hours, that is Perovskite structure oxide Sr can be prepared 0.9 Ce 0.1 CoO 3-δ The sample (SCCO010 for short), numbered No.2.
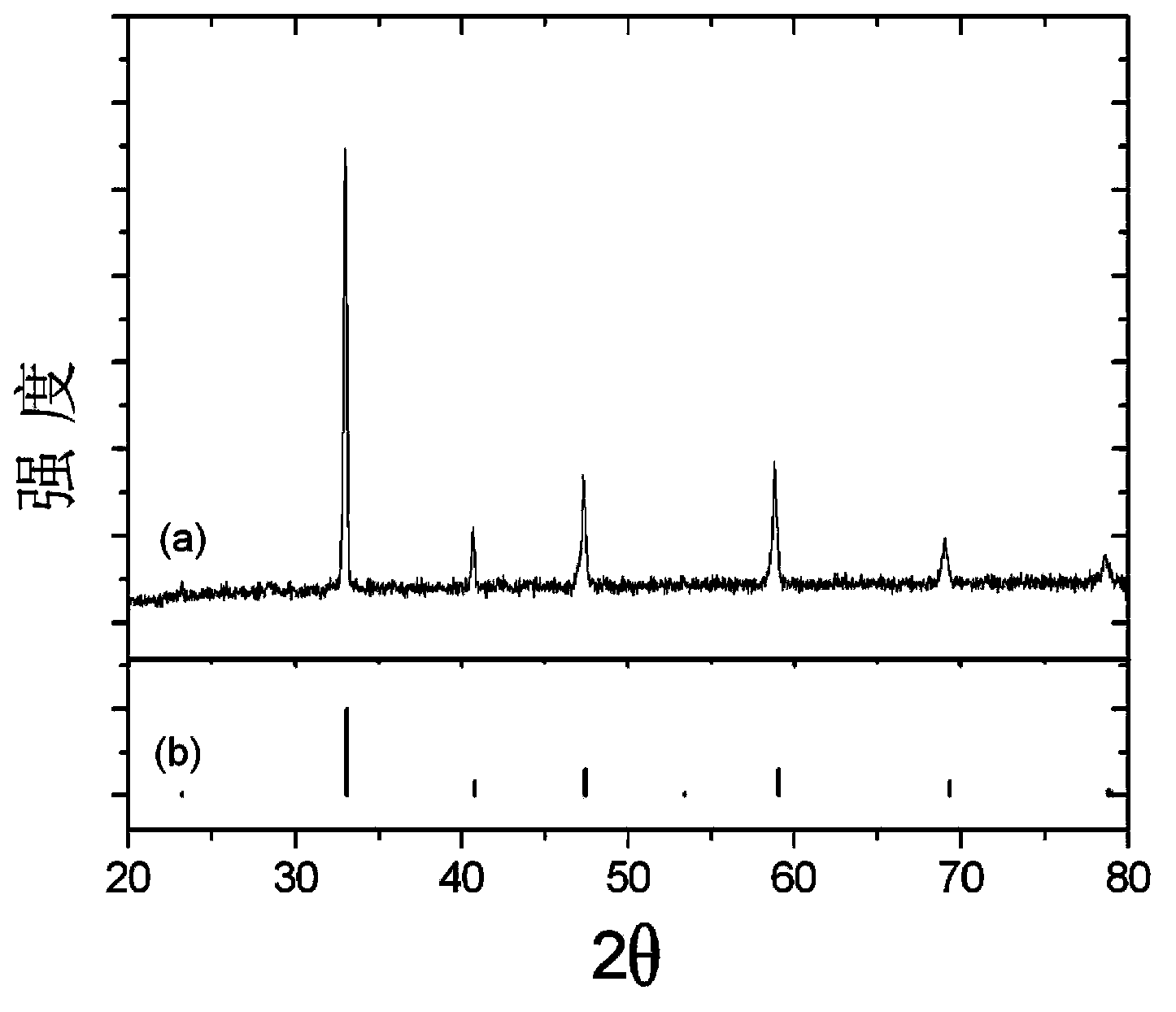
[0124] Adopt X-ray diffraction method to test the crystal structure of sample, its X-ray diffraction spectrum figure is similar to embodiment 1, and all diffraction peaks can be indexed as a pure tetragonal phase Sr 0.9 C...
Embodiment 3
[0126] This example is used to illustrate the perovskite structure oxide Sr 0.95 Ce 0.05 CoO 3-δ and oxygen ion conductor oxide Sm 0.2 Ce 0.8 o 1.9 (SDC) composite material Sr 0.95 Ce 0.05 CoO 3-δ - SDC and method for its preparation.
[0127] Composite Sr 0.95 Ce 0.05 CoO 3-δ -The concrete preparation method of SDC comprises:
[0128] According to the ratio of 7:3 by weight, the Sm prepared by burning glycine 0.2 Ce 0.8 o 1.9 (abbreviated as SDC, its particle size is about 20 nanometers) and the perovskite structure oxide Sr prepared in Example 1 0.95 Ce 0.05 CoO 3-δ The composite material Sr 0.95 Ce 0.05 CoO 3-δ -SDC (SCCO005+SDC for short), No.3.
[0129] Of course, this embodiment can also use the perovskite structure oxide Sr 0.95 Ce 0.05 CoO 3-δ and Sm 0.2 Ce 0.8 o 1.9 Mix evenly by mechanical grinding.
[0130] It should be pointed out that the purpose of the ball milling or mechanical grinding used in the present invention is to make the mech...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com