Construction method of recombinant plasmid of PD-L1 (programmed death-ligand 1) in chicken peripheral blood mononuclear lymphocytes, real-time gene abundance detection method and application of detection method
A technology of PD-L1 and lymphocytes, applied in the field of molecular pathology and immunology, to achieve the effect of enriching the mechanism of immunosuppression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Construction of chicken PD-L1 real-time fluorescent quantitative PCR positive standard recombinant plasmid
[0048] The peripheral blood of 4-week-old chicks was collected, and the lymphocytes in the peripheral blood were separated according to the instructions of the lymphocyte separation medium, and the total RNA was extracted according to the instructions of the Invitrogen TRIzol kit and related literature. The extracted total RNA was reverse-transcribed into cDNA according to the instructions of the reverse transcription kit, and stored at -20°C for use as a template for amplifying the target gene fragment of PD-L1.
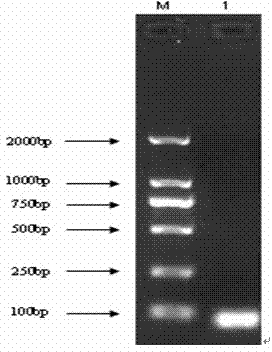
[0049] The target gene fragment of PD-L1 is amplified by ordinary PCR method, and the target gene fragment is detected by agarose gel electrophoresis, recovered and purified; then the purified fragment of the PD-L1 target gene is connected to the pMD 18-T vector, and transformed into a Extract the recombinant plasmid from DH5α cells in state,...
Embodiment 2
[0060] Example 2: Establishment of chicken PD-L1 real-time fluorescent quantitative PCR system
[0061] (1) Preparation of plasmid DNA template
[0062] The target gene fragment of PD-L1 was amplified by ordinary PCR amplification method, and the target gene fragment was detected by 2% agarose gel electrophoresis, recovered and purified, and transformed into competent cells after ligation with pMD 18-T vector (TaKaRa, Dalian) In DH5α, the recombinant plasmid was extracted; after clone screening, sequencing analysis was performed, and the sequencing result showed that it was 100% homologous to the sequence of the PD-L1 gene in GenBank, indicating that the obtained sequence was correct, and the positive plasmid could be used for the production of plasmid standards. The standard plasmid DNA concentration was determined to be 104.6 μg / mL using a micronucleic acid protein spectrophotometer, and the initial standard plasmid solution was diluted 1:10 times.
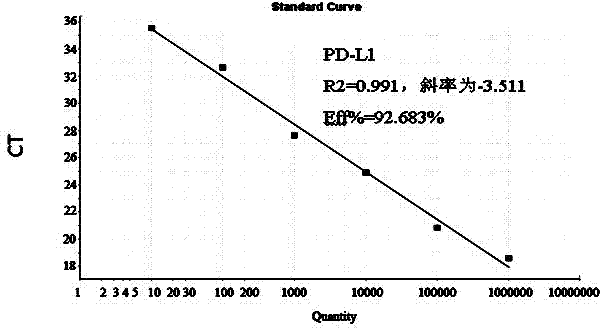
[0063] (2) Calculation ...
Embodiment 3
[0077] Example 3: Sensitivity, specificity and repeatability analysis of chicken PD-L1 real-time fluorescent quantitative PCR system
[0078] The chicken PD-L1 recombinant plasmid constructed in Example 1 was used as the positive recombinant standard plasmid; the chicken PD-L1 real-time fluorescent quantitative PCR detection system established in Example 2 was used; the real-time fluorescent quantitative PCR instrument was produced by Life Technologies (USA) ABI 7500 fluorescent quantitative PCR instrument was used.
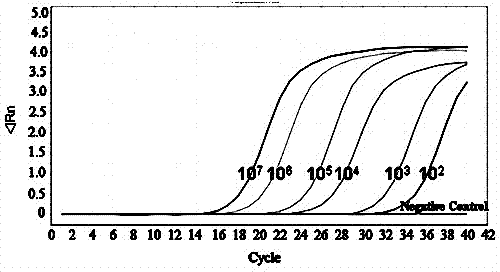
[0079] with 10-fold serial dilutions of 10 1 ~10 9 Copies / μL chicken PD-L1 positive recombinant standard plasmid for sensitivity test, the results showed that the detection limit of PD-L1 was 10 copies / μL.
[0080] Real-time-PCR melting curve for chicken PD-L1 molecule (see attached Figure 4 ) and product agarose gel electrophoresis for specificity analysis, the results showed that a single narrow peak appeared in the melting curve, and a specific fragment of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com