Method for producing alpha-ketoisocaproate by whole-cell transformation
A technology of ketoisocaproic acid and cells, which is applied in the field of fermentation engineering, can solve the problems of bacterial toxicity, difficulty in large-scale use, and high price, and achieve the effects of less environmental pollution, short production cycle, and fast reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1L-Amino acid deaminase recombinant plasmid construction
[0022] Using the genome of Proteus vulgaris as a template, with
[0023] 5'CGC GGATCC ATGGCGATATCTAGAAGAAAATTTA3' is the forward primer, with
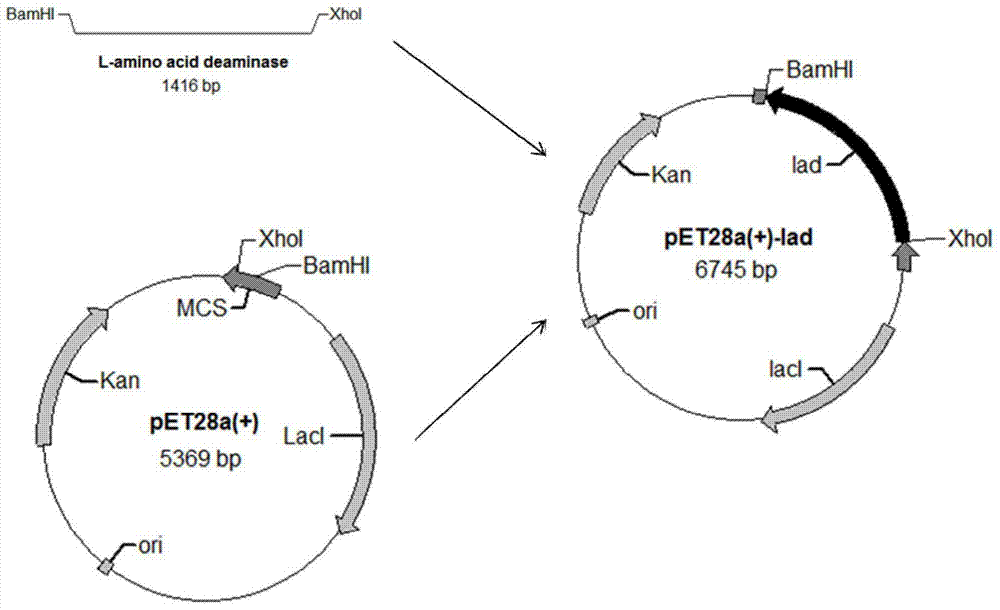
[0024] 5'CCG CTCGAG TTAGAATCTGTAAAGACTAAATGGTTT3' is the reverse primer, (the underlined parts are the restriction sites of BamH I and Xho I respectively) to amplify the target gene, and the nucleotide sequence of the target gene is shown in SEQ ID NO.1. After the PCR product was purified and digested, it was ligated with the vector pET28a(+) to construct the recombinant plasmid pET28a-lad (such as figure 1 ).
Embodiment 2
[0025] Embodiment 2 recombinant escherichia coli construction
[0026] The recombinant plasmid Pet28a-lad of Example 1 was transformed into the cloning host E.coli JM109, and a single colony grown on the LB ampicillin resistance plate was picked, PCR amplified, and positive transformants were selected to extract the plasmid, verified by double enzyme digestion, and the band Correct and sequenced, and transformed and expressed the host E.coli BL21(DE3) with the correct sequence.
Embodiment 3
[0027] Example 3 Condition optimization for whole cell conversion of L-leucine
[0028] Pick a single colony of recombinant Escherichia coli E.coli BL21(DE3) from the plate, inoculate it into the seed medium, cultivate it overnight, and inoculate it into 50mL fermentation medium with an inoculum of 2% (v / v), at 37°C , 200r / min culture to OD 600 0.4 to 1.5, add 0.4mM IPTG to induce, cultivate at 37°C for 5 hours, and collect the bacteria by centrifugation for whole cell transformation. With water as the reaction medium, conversion conditions:
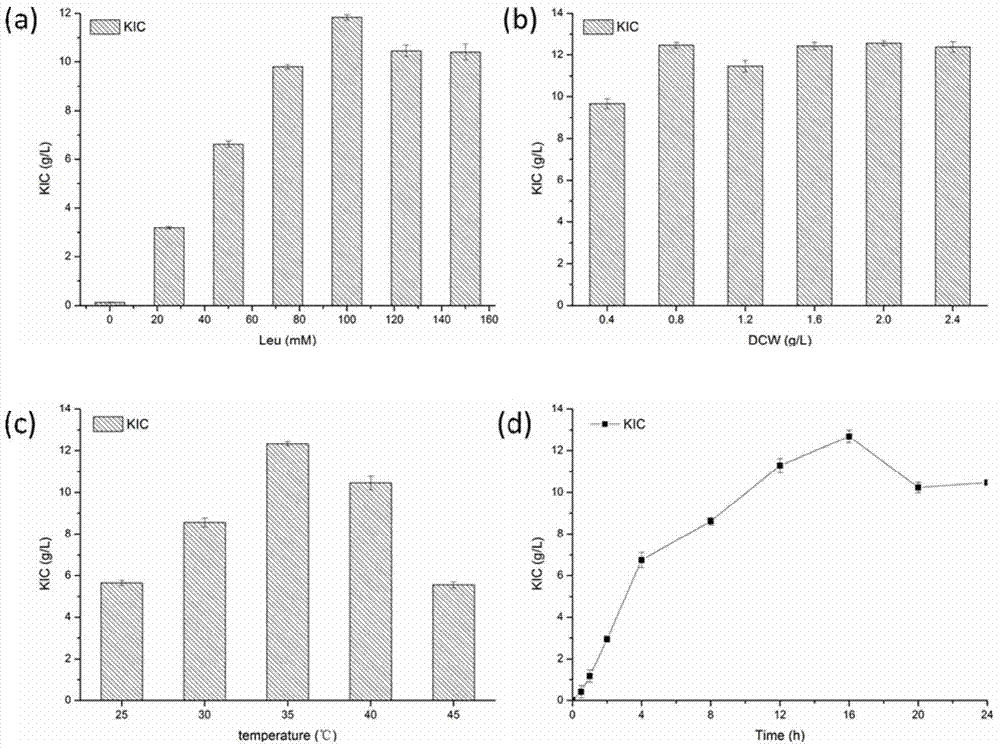
[0029] When the cell concentration is 1.0g / L, under the conditions of different concentrations of L-leucine (25mM, 50mM, 75mM, 100mM, 125mM, 150mM), transform at 37°C for 12h to investigate the effect of the substrate concentration on the yield. When the concentration of L-leucine was 100mM, the maximum yield reached 11.84g / L, and the conversion rate reached 90.95%.
[0030] When the concentration of L-leucine was 100mM, different con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com