Curcumin and piperine carried solid lipid nanoparticles and preparation method thereof
A solid lipid nano and solid lipid technology, applied in the field of pharmaceuticals, can solve the problems of limited clinical application, large toxic and side effects, etc., and achieve the effects of improving solubility, taking convenience, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] According to the emulsification evaporation-low temperature solidification method, 5 mg of curcumin, 5 mg of piperine, 100 mg of glyceryl monostearate, 150 mg of soybean lecithin, and 90 mg of oleic acid were precisely weighed respectively, and 5 mL of organic solvent (absolute ethanol: ethyl acetate = 3:2), heat and sonicate to dissolve, and heat and stir in a water bath after sonication to form an organic phase. Dissolve TPGS100mg and Brij78150mg in distilled water, heat and stir to form the water phase. Heat the organic phase and the water phase to about 60°C respectively, slowly pour the organic phase into the water phase under heating and stirring, and continue stirring after the organic solvent is completely volatilized. The obtained colostrum is rapidly cooled in a refrigerator at 4° C. to obtain a suspension of solid lipid nanoparticles.
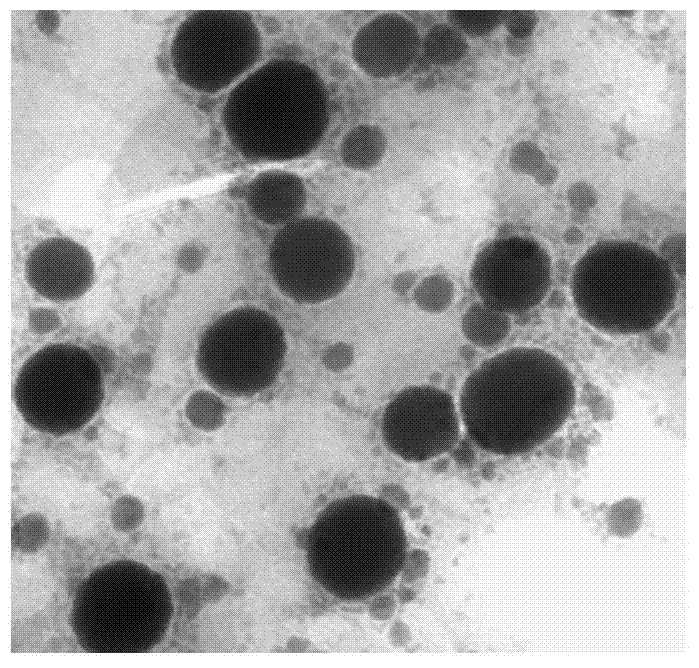
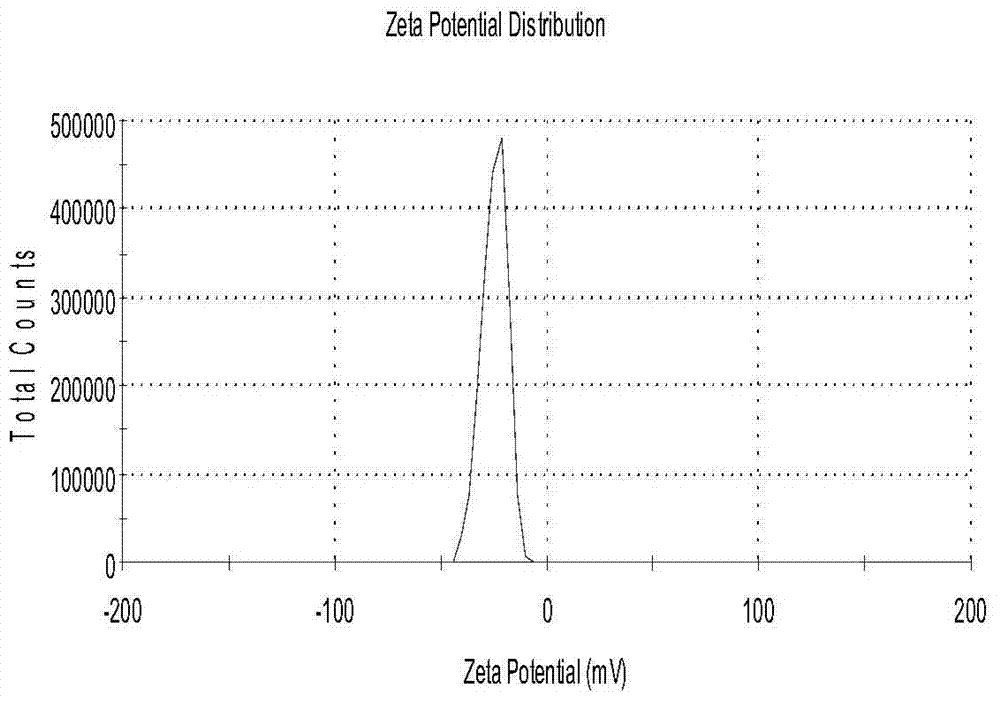
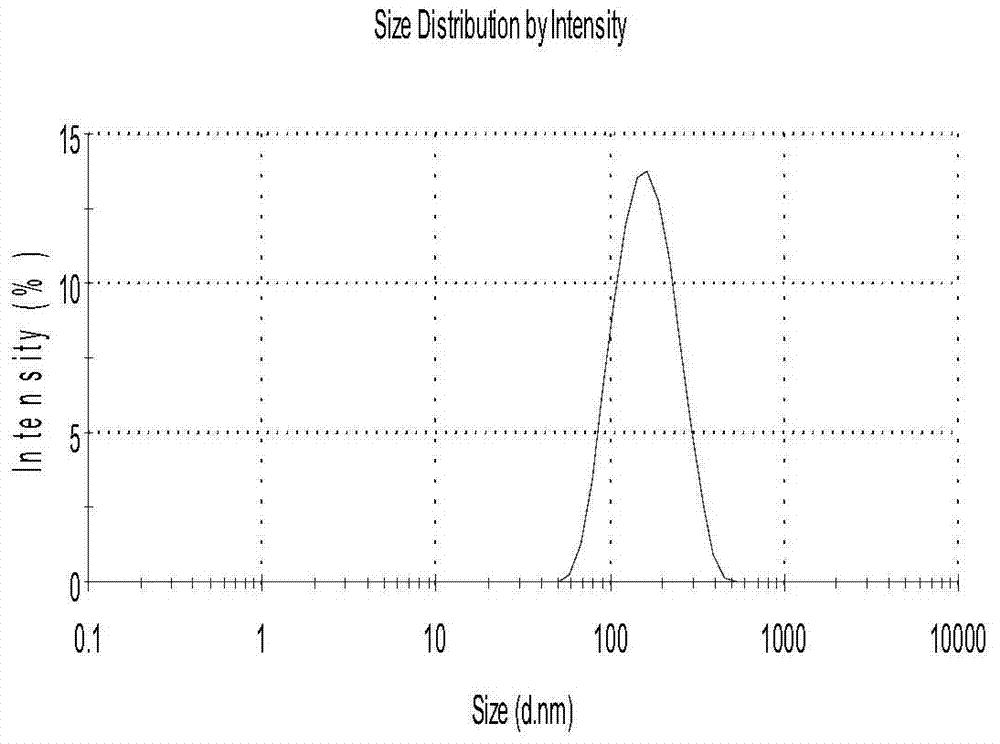
[0029]The physical and chemical properties of curcumin and piperine solid lipid nanoparticles obtained in Example 1 were st...
Embodiment 2
[0041] According to the microemulsion method, take 100 mg of glyceryl monostearate, 100 mg of egg yolk lecithin and 90 mg of oleic acid as the oil phase, 100 mg of TPGS and 850 mg of Brij as emulsifiers are placed in a glass bottle, heated to melt the oil phase, and ultrasonically stirred to emulsify it. Weigh 10 mg of curcumin and 5 mg of piperine, dissolve in 2 mL of absolute ethanol, transfer to a glass bottle containing the oil phase and emulsifier, heat and stir to remove absolute ethanol, and add preheated distilled water. Continue heating and stirring to form an oil-in-water microemulsion, and directly cool the formed drug-loaded nanoparticles to room temperature to obtain curcumin and piperine solid lipid nanoparticles.
[0042] The physical and chemical properties of the obtained curcumin and piperine solid lipid nanoparticles were studied, and the results showed that its average particle size was 168.5nm; the Zeta potential was-20.1mV; the encapsulation efficiency of ...
Embodiment 3
[0044] According to the film dispersion method, weigh 10 mg of curcumin, 5 mg of piperine, 100 mg of glyceryl monostearate, 200 mg of soybean lecithin, and 110 mg of oleic acid, and add 5 mL of organic solvent (absolute ethanol: ethyl acetate = 2:1) In , the water bath is heated and ultrasonicated to dissolve and form the organic phase. Transfer the organic phase to a round-bottomed flask, control the temperature of the water bath to about 60°C, and keep a proper angle between the round-bottomed flask and the horizontal plane. Turn on the vacuum pump to extract the air in the system, so that the organic solvent is completely evaporated, and the film is formed by rotary evaporation. Take emulsifier TPGS90mg and Brij78120mg and dissolve in distilled water to form the water phase. The aqueous phase was added to a round bottom flask and spin eluted. Transfer the suspension to a beaker, and after ultrasonication in a water bath, place it in a refrigerator at 4°C to cool and solid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com