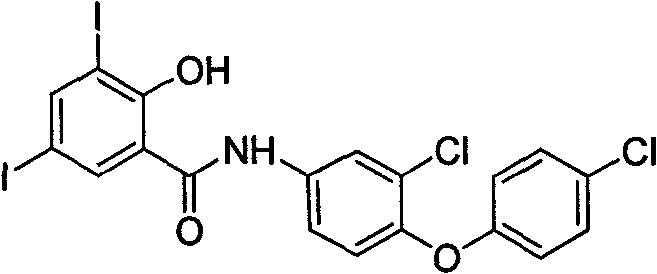
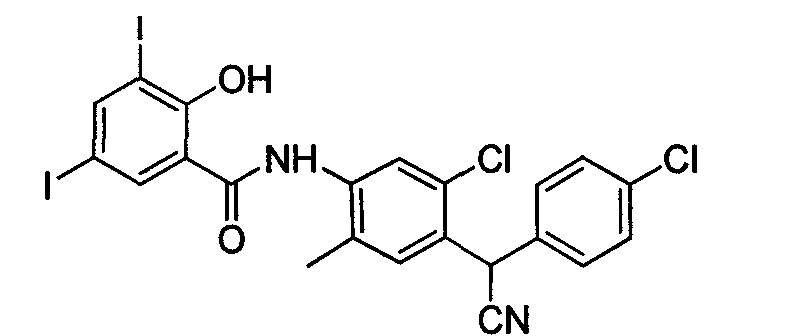
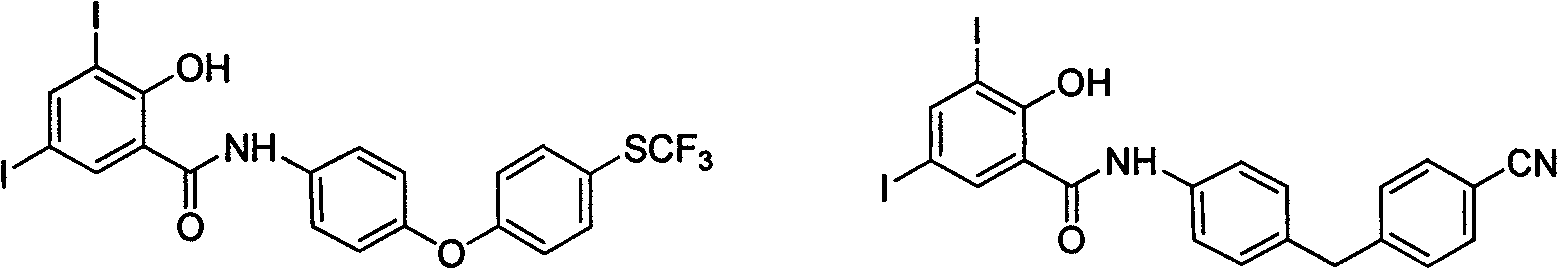
Poly-halogenated benzoic acid synthesizing method
A technology of polyhalogenated benzoic acid and halogenated benzoic acid is applied in the directions of organic chemical methods, chemical instruments and methods, organic halogenation, etc., and can solve problems such as unfavorable industrialized production, environmental pollution, environmental pollution, etc., and achieve cost saving of raw materials , the effect of shortening the reaction time and reducing the production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Into a 2L four-neck round bottom flask equipped with mechanical stirring, thermometer and condenser, 138.1g (1.00mol) of salicylic acid, 163.9g (1.03mol) of bromine, 7g (0.04 mol) was dissolved in methanol (500ml), stirred and heated to 45°C, 175g (1.5mol) of 30% hydrogen peroxide was added dropwise, the temperature was controlled at 55-60°C, and the dropping time was 1 hour. After dripping, keep it at 65°C for 4 hours. Then 500 g of water was added, the water bath was lowered to room temperature, filtered, and dried to obtain 277.6 g of 3,5-dibromosalicylic acid, with a yield of 93.8%. Melting point 224-226°C; 1 H NMR(DMSO-d 6 , 400MHz): δ 7.79 (d, J=2.4 Hz, 1H, ArH), 7.94 (d, J=2.4 Hz, 1H, ArH), 10.36 (s, 1H, OH).
Embodiment 2
[0040] Example 2: In a 2L four-neck round bottom flask equipped with mechanical stirring, thermometer and condenser, 138.1g (1.00mol) of salicylic acid, 261.4g (1.03mol) of iodine and 7g (0.04mol) of trichloroacetic acid were added Dissolve in methanol (500ml), stir and raise the temperature to 45°C, add 175g (1.5mol) of 30wt% hydrogen peroxide dropwise, control the temperature during the dropping at 60-65°C, and the dropping time for 1 hour. After dripping, keep it at 66°C for 5 hours. 500 g of water was added, the water bath was lowered to room temperature, filtered and dried to obtain 374.1 g of 3,5-diiodosalicylic acid, with a yield of 95.9%. Melting point: 233-235℃; 1 H NMR(DMSO-d 6 , 400MHz): δ 8.024 (d, J=2.4 Hz, 1H, ArH), 8.238 (d, J=2.4 Hz, 1H, ArH).
Embodiment 3
[0041] Example 3: In a 2L four-necked round bottom flask equipped with mechanical stirring, thermometer and condenser, 137.1g (1.00mol) of 2-aminobenzoic acid, 261.4g (1.03mol) of iodine, 7g (0.04 mol) was dissolved in methanol (500ml), stirred and heated to 45°C, 175g (1.5mol) of 30wt% hydrogen peroxide was added dropwise, the temperature was controlled at 60-65°C, and the dropping time was 1 hour. After dripping, keep it at 68°C for 5 hours. 500 g of water was added, the water bath was lowered to room temperature, filtered and dried to obtain 350.7 g of 2-amino-3,5-diiodobenzoic acid with a yield of 90.2%. Melting point: 232-234°C; 1 H NMR(DMSO-d 6 , 400MHz): δ 6.31 (s, 2H, NH2), 7.87 (d, J=2.4 Hz, 1H, ArM), 8.06 (d, J=2.4 Hz, 1H, ArH).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com