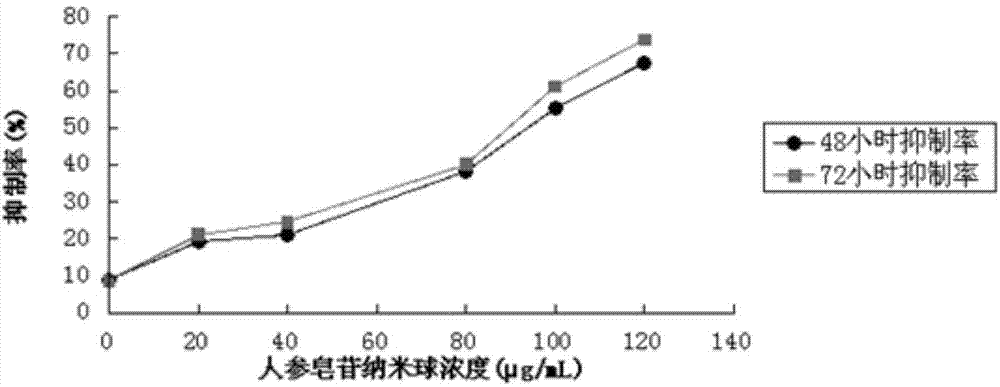
Complex nano particle of ginsenoside Rh2 albumin and preparation method thereof
A technology of ginsenosides and human serum albumin, which is applied in the directions of pharmaceutical formulations, drug combinations, and medical preparations with inactive ingredients, etc., can solve the problems of low bioavailability, large particles, and insolubility in water.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The albumin composite nanoparticles of ginsenoside Rh2 involved in the present invention are prepared through the following steps:
[0021] Preparation of albumin composite nanoparticles of ginsenoside Rh2: Dissolve ginsenoside Rh2 in a small amount of absolute ethanol, weigh 250 mg of albumin and dissolve it in 25 ml deionized water, add ginsenoside Rh2 after complete dissolution, and place on a magnetic stirrer Mix well, adjust the pH to be slightly alkaline (pH9.0), slowly (drop by drop about 1ml / 1min) add an appropriate amount of absolute ethanol, stir for half an hour until the solution becomes turbid, then slowly add about 50ul of glutaraldehyde solution, Continue to stir and solidify for 12 hours, centrifuge the reaction solution in a centrifuge tube (21000rpm) for half an hour, wash with water three times, filter through 220um, vacuum dry and store at 4°C for later use.
[0022] The optimal conditions were explored through various conditions such as the ratio of...
Embodiment 2
[0031] Example 2: The effect of absolute ethanol amount and the ratio pH of the aqueous phase on the stability of albumin composite nanoparticles of ginsenoside Rh2
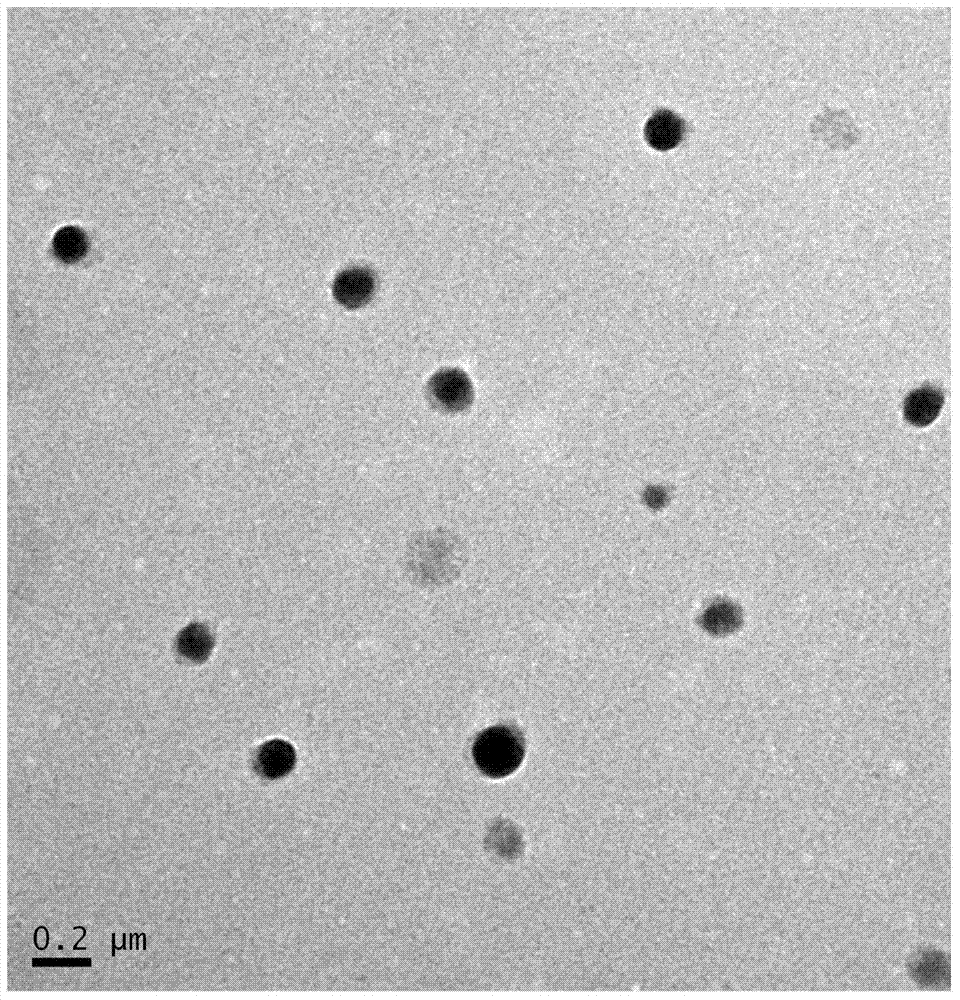
[0032] Weigh 250mg of albumin and dissolve it in 25ml of deionized water, add ginsenoside Rh2 after completely dissolving, mix well on a magnetic stirrer, slowly (drop by drop about 1ml / 1min) add about 150ml of absolute ethanol to prepare ginsenoside Rh2 white Compared with the nanoparticles added with 100ml and 200ml of absolute ethanol, the protein composite nanoparticles have a more uniform particle size and a moderate size, which is suitable for relevant animal experiments and clinical use, and has a higher encapsulation efficiency and drug loading capacity.
Embodiment 3
[0033] Example 3: Effect of buffer pH on the stability of albumin composite nanoparticles of ginsenoside Rh2
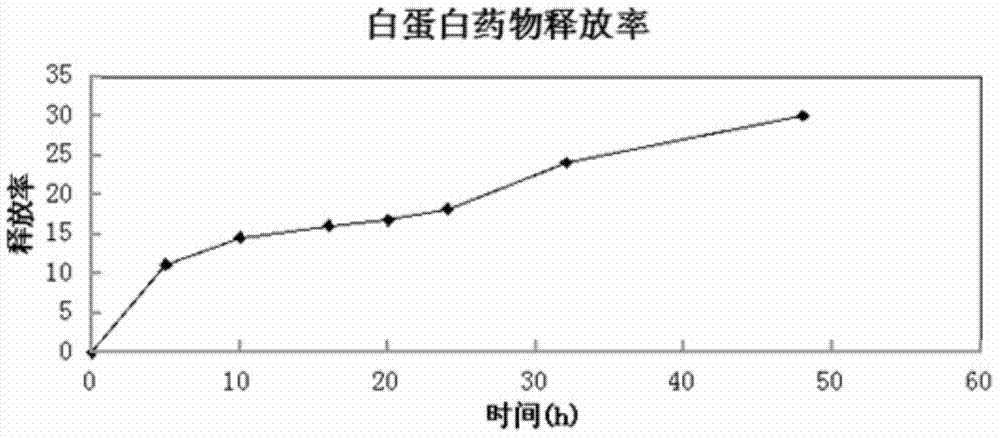
[0034] Using pH 9.0 deionized aqueous solution to reconstitute this product, in terms of encapsulation efficiency and particle size, there is no significant difference between pH 7.0 and 5.0. However, after several months of storage at 4°C, the drug release rate was significantly different from that at pH 7.0 and 5.0. The product reconstituted at pH 5.0 is easy to settle and is not suitable for long-term storage.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com