Method for preparing high-temperature inorganic scintillation crystal
A scintillation crystal and high-temperature technology, which is applied in the field of high-temperature inorganic scintillation crystal preparation, can solve the problems of large fluctuations in crystal scintillation performance, high price, and high crystal growth cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Example 1: Crystal growth of cerium-doped lutetium silicate
[0012]1. Synthesis of raw materials by solid-phase raw material synthesis method
[0013] Assuming the synthesis of Ce doped with cerium ion concentration x 2x :Lu 2(1-x) SiO 5 The chemical cooperation reaction formula for sintering of polycrystalline raw materials and solid phase raw materials is:
[0014] 2x CeO 2 +(1-x)Lu 2 o 3 +SiO2 2 = Ce 2x :Lu 2(1-x) SiO 5 +x / 2O 2 ↑
[0015] If it is planned to prepare raw materials with an active ion concentration of 0.5mol%, then x=0.5mol%, according to the molar ratio of 0.01:0.995:1, respectively weigh CeO with a purity of 99.95% 2 、Lu 2 O3 and SiO 2 powder raw material.
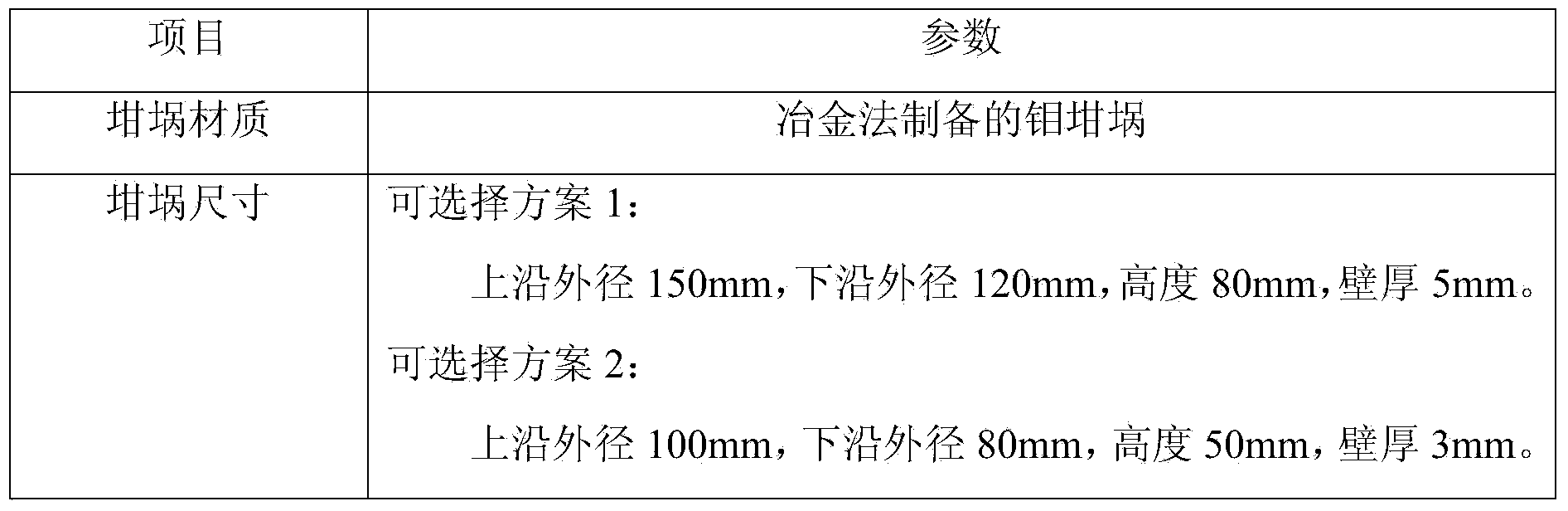
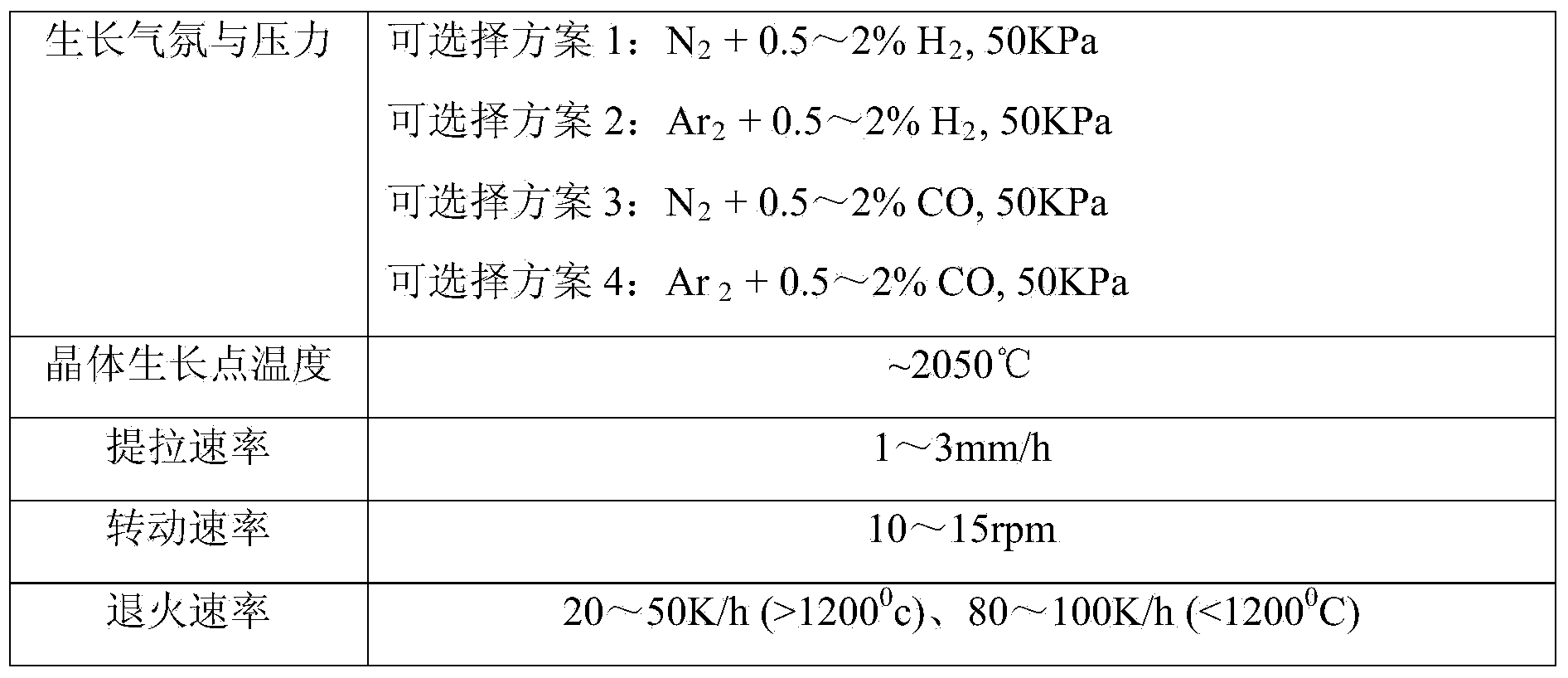
[0016] Put the three raw materials into an agate tank, and mix them on the mixer for 12 hours to ensure that the three components are evenly mixed; then add a small amount of pure water, and use hydraulic equipment to press the mixed raw materials into a 80mm in diameter and 20mm i...
Embodiment 2
[0024] Embodiment 2: Crystal growth of cerium-doped yttrium-lutetium silicate
[0025] Assuming that the synthesis of Ce doped with cerium ion concentration x and yttrium ion concentration y 2x :Y 2y Lu 2(1-x-y) SiO 5 The chemical cooperation reaction formula for sintering of polycrystalline raw materials and solid phase raw materials is:
[0026] 2x CeO 2 +y Y 2 o 3 +(1-x-y) Lu 2 o 3 +SiO2 2 = Ce 2x :Y 2y Lu 2(1-x-y) SiO 5 +x / 2O 2 ↑
[0027] If the concentration of activated ions is planned to be 0.5mol%, and the doping ratio of yttrium ions is 10mol%, then x=0.5mol%, y=10mol%, according to the molar ratio of 0.01:0.1:0.895:1, the purity is 99.95 %CeO 2 , Y 2 o 3 、Lu 2 o 3 and SiO 2 powder raw material.
[0028] Subsequent parameters and steps of polycrystalline raw material sintering and crystal growth are consistent with Embodiment 1.
Embodiment 3
[0029] Embodiment 3: Crystal growth of cerium-doped lutetium aluminum garnet
[0030] Assuming the synthesis of Ce doped with cerium ion concentration x 3x :Lu 3-3x Al 5 o 12 The chemical cooperation reaction formula for sintering of polycrystalline raw materials and solid phase raw materials is:
[0031] 6x CeO 2 +3(1-x) Lu 2 o 3 +5 Al 2 o 3 =2Ce 3x :Lu 3-3x Al 5 o 12 +4.5O 2 ↑
[0032] If the planned concentration of activated ions is 0.5mol%, then x=0.5mol%, according to the molar ratio of 0.03:2.985:5, respectively weigh CeO with a purity of 99.95% 2 、Lu 2 o 3 and Al 2 o 3 powder raw material.
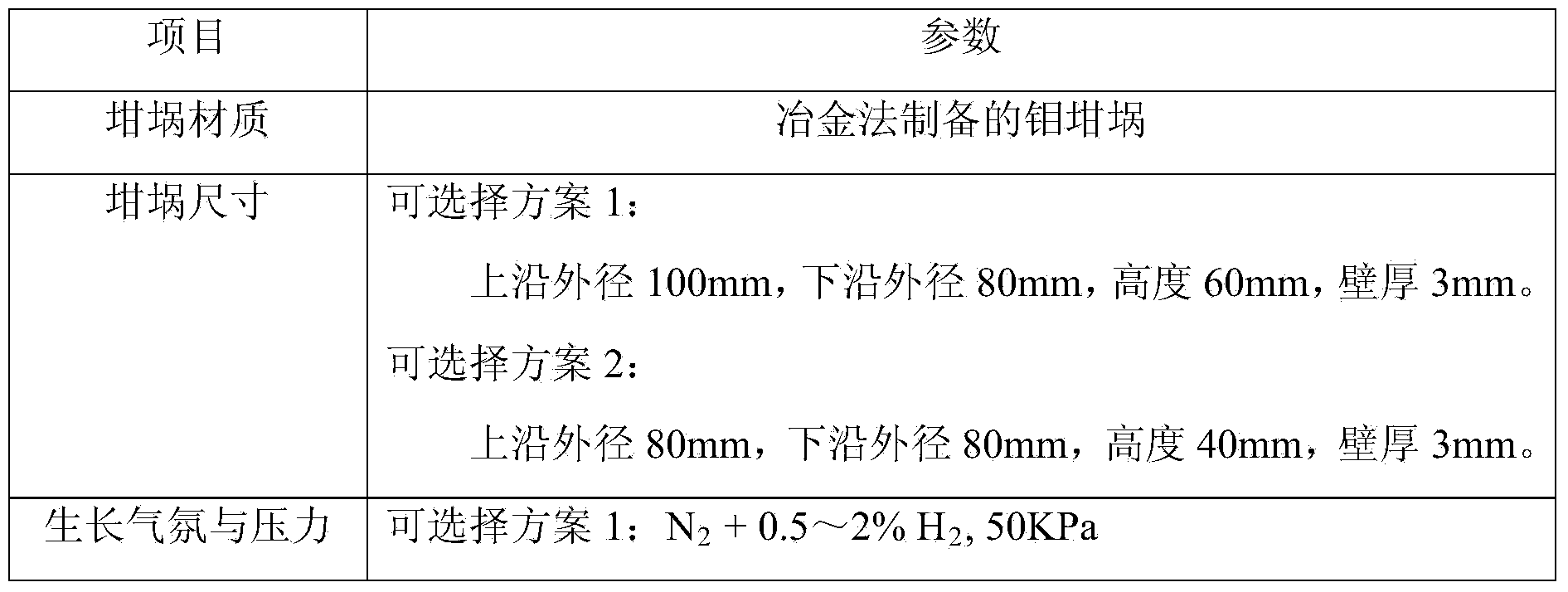
[0033] Put the three raw materials into an agate tank, and mix them on the mixer for 12 hours to ensure that the three components are evenly mixed; then add a small amount of pure water, and use hydraulic equipment to press the mixed raw materials into a 60mm in diameter and 10mm in thickness Cylindrical block of raw material. Put the raw material block into a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com