Anti-carbon deposition nimethyl methane steam reforming hydrogen production catalyst and preparation method thereof
A technology for producing hydrogen from methane steam and reforming, which is applied in the field of catalysis, can solve the problems of insufficient catalytic activity and stability, and achieve the effects of strong anti-carbon deposition ability, cost reduction and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Ni / La 2 Zr 2 o 7 Particle catalyst, the preparation method is as follows:
[0032] (1) La(NO 3 ) 3 ·6H 2 O, Zr(NO 3 ) 3 ·3H 2 O is dissolved in metered deionized water to form a solution with a concentration of 0.5mol / L;
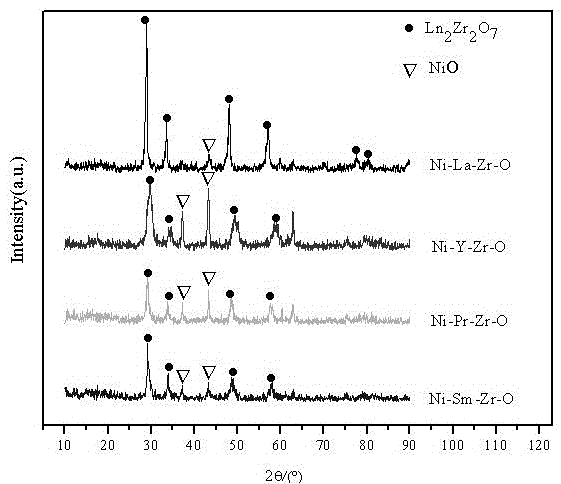
[0033] (2) Dilute the industrial ammonia water with a concentration of 25% to 1 times as a precipitating agent. Under continuous stirring, add the salt solution obtained in (1) dropwise to the above precipitating agent, control the pH=10, and after the precipitation is complete, let it stand at room temperature. After 12 hours, wash with deionized water until the TDS of the filtrate2 Zr 2 o 7 Composite oxide support, characterized by XRD, such as figure 1 As shown, the support has pyrochlore-type structure characteristic diffraction peaks; X-ray diffraction results show that catalysts with pyrochlore-type structures containing different rare earths have been synthesized.
[0034] (3) Ni(NO) with a nickel content of 12% catalyst mass 3 ) ...
Embodiment 2
[0037] Ni / Y 2 Zr 2 o 7 Particle catalyst, the preparation method is as follows:
[0038] (1) Y(NO) with Y:Zr (molar ratio) of 1:1 3 ) 3 ·6H 2 O, Zr(NO 3 ) 3 ·3H 2 O is dissolved in metered deionized water to form a solution with a concentration of 0.5mol / L;
[0039] (2) Dilute the industrial ammonia water with a concentration of 25% to 1 times as a precipitating agent. Under continuous stirring, add the salt solution obtained in (1) dropwise to the above precipitating agent, control the pH=10, and after the precipitation is complete, let it stand at room temperature. After 12 hours, wash with deionized water until the TDS of the filtrate2 Zr 2 o 7 Composite oxide support, characterized by XRD, such as figure 1 As shown, the carrier has pyrochlore-type structure characteristic diffraction peaks;
[0040] (3) Ni(NO) with a nickel content of 12% catalyst mass 3 ) 3 ·6H 2 O was dissolved in metered deionized water, and the prepared carrier was impregnated in metered...
Embodiment 3
[0043] Ni / Pr 2 Zr 2 o 7 Particle catalyst, the preparation method is as follows:
[0044] (1) Pr(NO 3 ) 3 ·6H 2 O, Zr(NO 3 ) 3 ·3H 2 O is dissolved in metered deionized water to form a solution with a concentration of 0.5mol / L;
[0045] (2) Dilute the industrial ammonia water with a concentration of 25% to 1 times as a precipitating agent. Under continuous stirring, add the salt solution obtained in (1) dropwise to the above precipitating agent, control the pH=10, and after the precipitation is complete, let it stand at room temperature. After 12 hours, wash with deionized water until the TDS of the filtrate2 Zr 2 o 7 Composite oxide support, characterized by XRD, such as figure 1 As shown, the carrier has pyrochlore-type structure characteristic diffraction peaks;
[0046] (3) Ni(NO) with a nickel content of 12% catalyst mass 3 ) 3 ·6H 2 O was dissolved in metered deionized water, and the prepared carrier was impregnated in metered nickel salt solution by equal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com