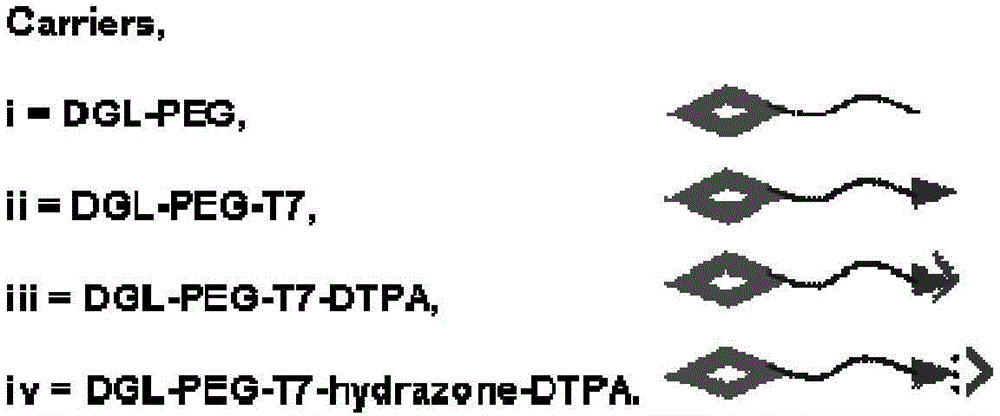
A kind of tumor active targeting nano-drug delivery system and preparation method thereof controlled by slightly acidic environment
An active targeting, slightly acidic environment technology, applied in antitumor drugs, pharmaceutical formulations, drug combinations, etc., to achieve the effects of improving targeting efficiency, simple preparation method, high targeting and treatment efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Dissolve polylysine in an appropriate amount of methanol to prepare a 10mg / ml solution, take 100μl and dry it in a vial; dissolve polyethylene glycol maleimide-polyethylene glycol 3500-succinimide in An appropriate amount of 0.035MpH 8.0 phosphate buffer solution was prepared to make a 5mg / ml working solution, and 318μl polyethylene glycol working solution was added to a vial, stirred and reacted at room temperature for 2h, and polylysine-polyethylene glycol DGL- PEG was added into a MWCO 5000 ultrafiltration centrifuge tube, purified by ultrafiltration at 12000 rpm for 30 minutes, and the unmodified carrier polylysine-polyethylene glycol DGL-PEG was obtained.
Embodiment 2
[0051] Dissolve polylysine in an appropriate amount of methanol to prepare a 10mg / ml solution, take 100μl and dry it in a vial; dissolve polyethylene glycol maleimide-polyethylene glycol 3500-succinimide in Prepare an appropriate amount of 0.035MpH 8.0 phosphate buffer solution to prepare a 5mg / ml working solution, take 318μl polyethylene glycol working solution and add it to a vial, stir and react at room temperature for 2h; then dissolve T7 in an appropriate amount of pH 7.0 phosphate buffer solution , prepared into a 1 mg / ml polypeptide solution, took 226 μl and added it to the polylysine-polyethylene glycol solution, stirred and reacted at room temperature for 24 hours, added it to a MWCO 5000 ultrafiltration centrifuge tube, purified it by ultrafiltration at 12000 rpm for 30 minutes, and obtained The carrier polylysine-polyethylene glycol-polypeptide DGL-PEG-T7 modified by targeting polypeptide T7.
Embodiment 3
[0053] Weigh 28.2 mg DGL-PEG-T7 (0.454 μmol) and 5.9 mg p-SCN-Bn-DTPA (9.08 μmol) in 12 mL PBS 9.0 for 48 hours at room temperature. The hydrazide at the end of the polypeptide T7 on the carrier can first undergo an addition reaction with the isothiocyanate on DTPA, and connect DTPA to the end of the polypeptide T7 on the carrier through an amide bond to obtain DPTD; remove unreacted p-SCN-Bn-DTPA.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com