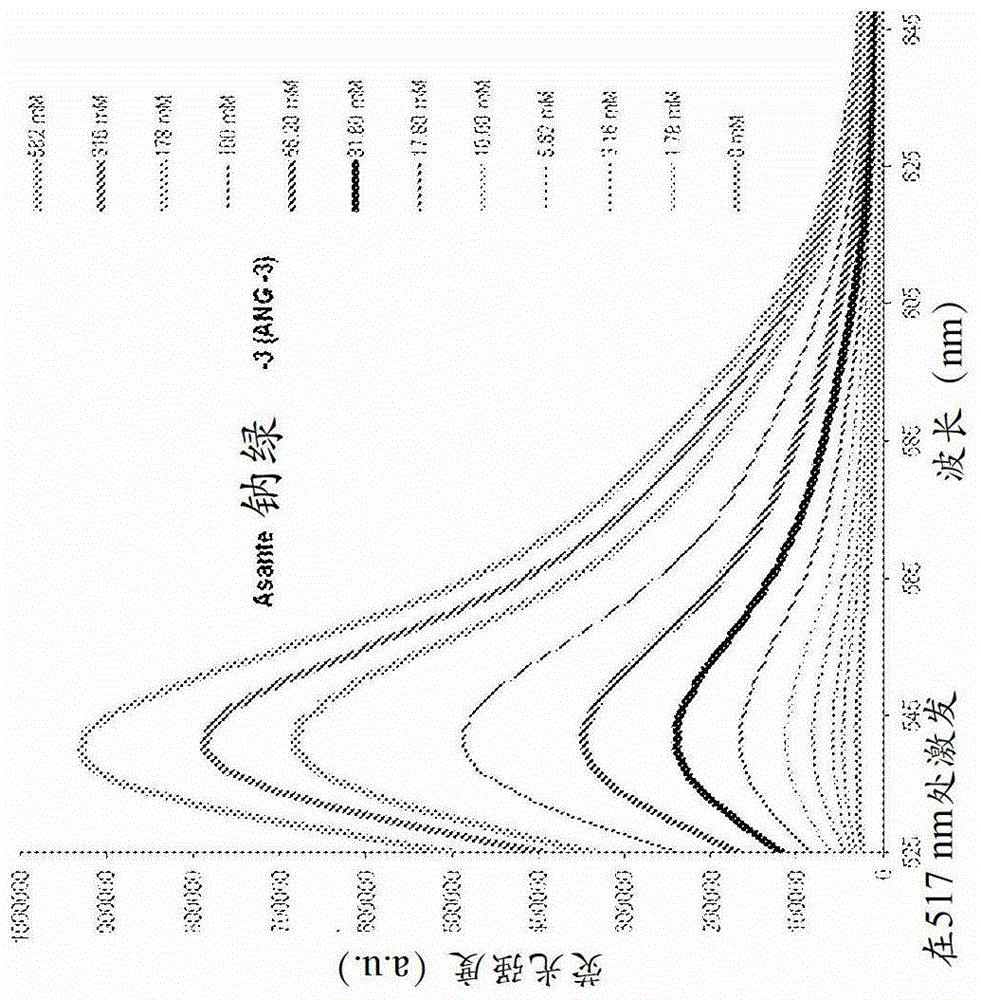
cytoplasmic fluorescent ion indicator
An indicator and fluorescent technology, applied in the field of fluorophores, can solve problems such as poor water solubility and limited organic solvent ion measurement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example to , approach 9
[0267]
[0268] O-anisidine (362 mL) was added along with diisopropylethylamine (233 mL) and 1,2-bis(2-chloroethoxy)ethane (84 mL). The reaction was stirred at 120 °C for 3 days. The crude product was placed on a rotary evaporator with an oil bath and heated to >100°C with high vacuum to remove most of the excess anisidine. After this time, the residue was diluted with ethyl acetate and washed twice with water, then dried over sodium sulfate and evaporated. The crude product was evaporated under vacuum and purified by column chromatography using 6:1 hexane / ethyl acetate with increasing ethyl acetate content.
[0269]
[0270] Diglycolic acid (25 g) was added to a dry flask. To this was added thionyl chloride (85 mL) and the reaction was immediately heated to reflux and stirred at this temperature for 5 hours. Simultaneously, the excess thionyl chloride was distilled at 85° C. under reduced pressure, then at room temperature by high vacuum until the mixture precipitate...
example 1
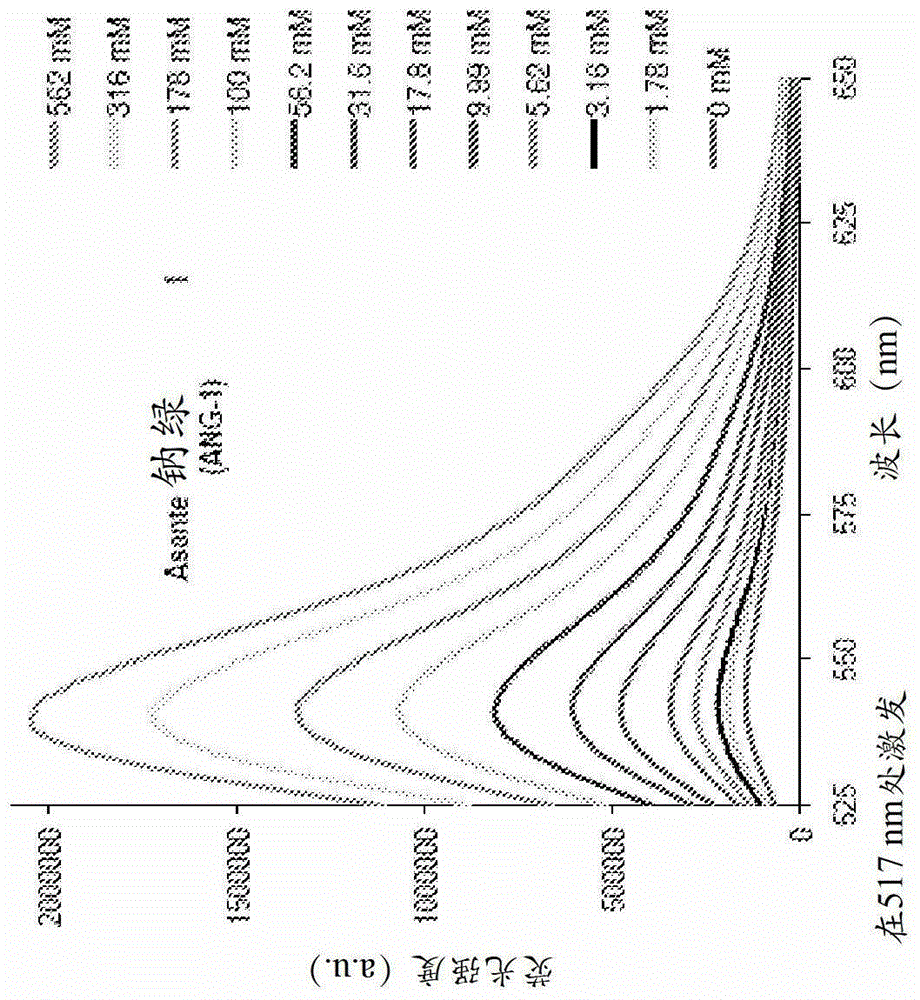
[0285] Example 1 - Sodium indicator ANG-1
[0286] figure 1 is the sodium-titrated fluorescence emission spectrum of Asante NatriumGreen-1 (ANG-1) salt solution (1M TMACl, 10 mM MOPS, pH=7.10) titrated with 1M sodium chloride solution excited at 517 nm.
[0287] Figure 7 It is shown that HEK293 cells loaded with ANG-1 (AM) express TRPV1 channels. Capsaicin, an agonist of the TRPV1 channel, conducts Na + and Ca 2+ , was used to excite Na + inflow.
[0288] Figure 8 Shown are REF52 cells loaded with ANG-1(AM) and applied Na + The ionophore gramicidin to promote Na + inflow. The obtained Na + An increase in the intracellular concentration resulted in a corresponding increase in ANG-1 fluorescence.
[0289] Figure 9 Shown are astrocytes loaded with ANG-1 (AM), showing Na due to ouabain inhibition of this sodium pump + An increase in intracellular concentration results in an increase in fluorescence from the first box to the second box.
example 2
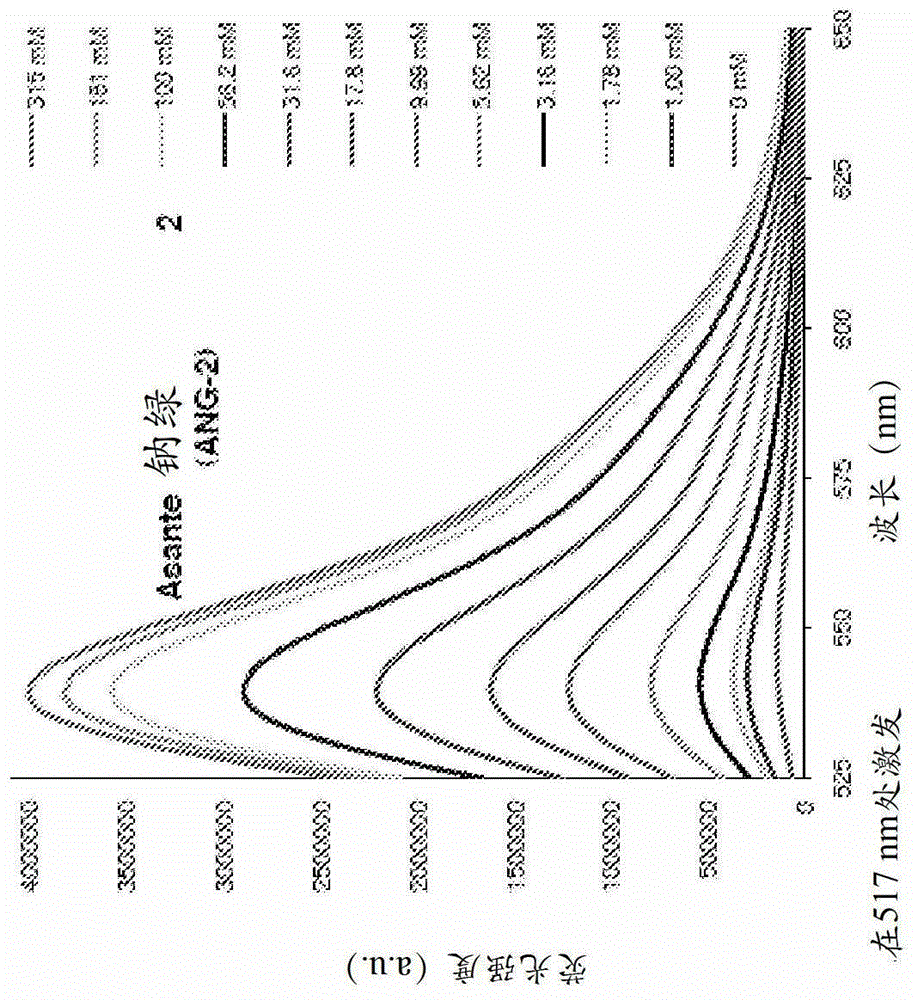
[0290] Example 2-sodium indicator ANG-2
[0291] figure 2 It is Asante Natrium Green (sodium green)-2 (ANG-2) salt solution (140mM TMACl, 10mM MOPS, pH=7.10) titrated with sodium chloride solution (1M NaCl, 10mM MOPS, pH=7.10) excited at 517nm 7.11), sodium titrated fluorescence emission spectrum.
[0292] Figure 10 Shown are REF52 fibroblasts loaded with ANG-2(AM) and the Na + ionophore SQI-Pr to promote Na + inflow. The obtained Na + An increase in the intracellular concentration resulted in a corresponding increase in ANG-2 fluorescence. Further addition of 20 μΜ amphotericin B gave only a weak increase in fluorescence.
[0293] Figure 11 Shown is REF52 fibroblasts loaded with ANG-2(AM) maintained in 145 mM NMG-gluconate. 50 μM Amphotericin-B depletes Na + and K + of these cells. Following equilibration of intracellular and extracellular sodium concentrations, incremental NaCl was added extracellularly, resulting in a corresponding increase in ANG-2 fluores...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com