Pharmaceutical compositions containing thermally stable oxygen carriers for different therapeutic applications
一种热稳定、组合物的技术,应用在药物组合、抗肿瘤药、医药配方等方向,能够解决无法接受高百分比的二聚体单位、血红蛋白组合物不能令人满意地施用哺乳动物等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
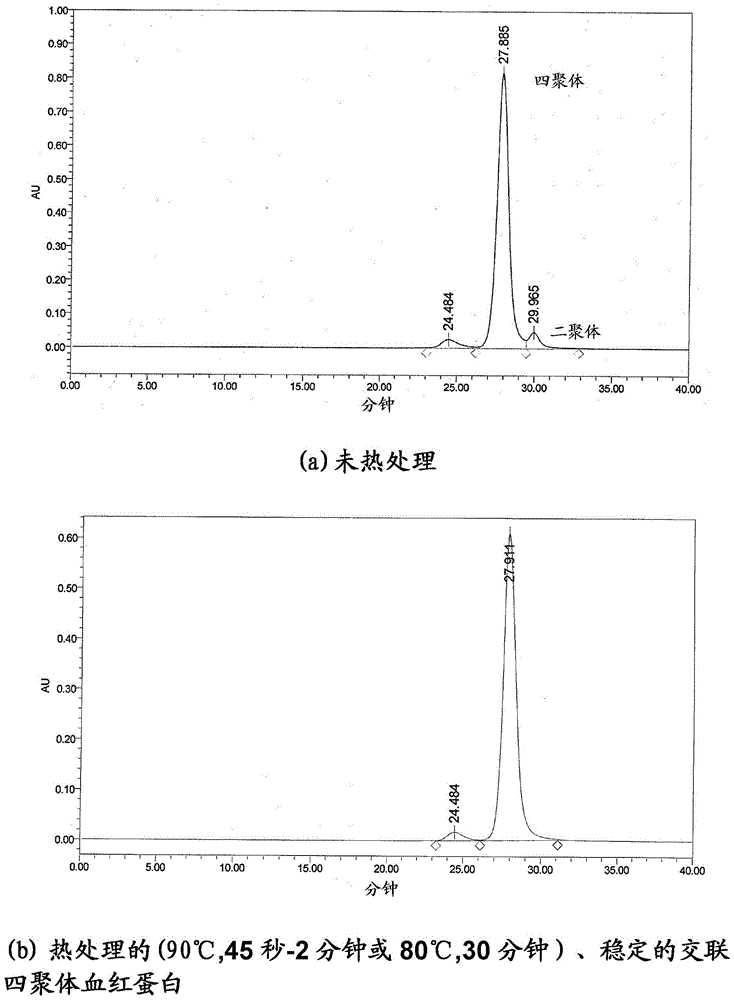
[0081] Process overview
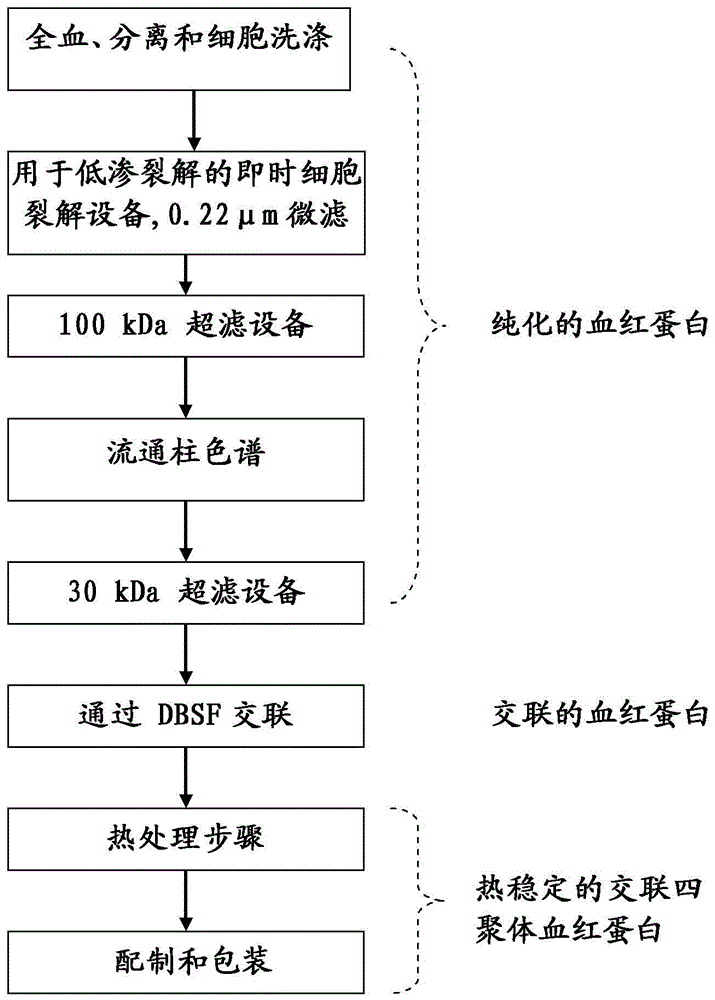
[0082] A schematic flow diagram of the method of the present invention is shown in figure 1middle. Bovine whole blood was collected in closed sterile containers / bags containing 3.8% (w / v) trisodium citrate solution as anticoagulant. The blood is then mixed immediately and thoroughly with trisodium citrate solution to inhibit blood clotting. Red blood cells (RBCs) are separated from plasma and other smaller blood cells and collected by apheresis mechamism. For this step, a "cell washer" was used with gamma sterilized disposable centrifuge bowls. RBCs were washed with an equal volume of 0.9% (w / v NaCl) saline.
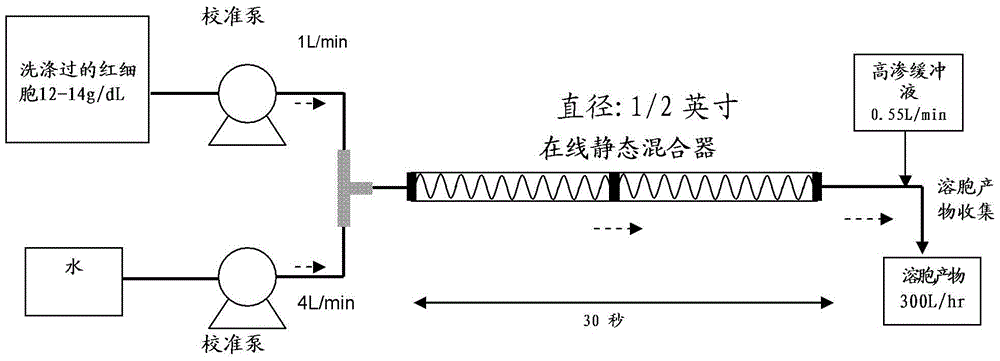
[0083] Washed red blood cells are lysed to release the hemoglobin contents by subjecting the red blood cell membrane to a hypotonic shock. figure 2 The dedicated point-of-care cell lysis device for the RBC lysis device drawn in was used for this purpose. After RBC lysis, hemoglobin molecules were separated from other proteins by tangentia...
Embodiment 2
[0086] Timed and controlled hypotonic lysis and filtration
[0087] Fresh bovine whole blood was collected and shipped under chilled conditions (2-10°C). Red blood cells were separated from plasma by a cell washer, followed by 0.65 μm filtration of red blood cells. After washing the red blood cell (RBC) filtrate with 0.9% saline, the filtrate was disrupted by hypotonic lysis. by using figure 2 The instant cell lysis device drawn in is used for hypotonic lysis. The instant cell lysis device includes a static mixer to aid in cell lysis. An RBC suspension containing controlled hemoglobin concentration (12-14 g / dL) was mixed with 4 volumes of purified water to generate a hypotonic shock to the RBC cell membranes. The period of hypotonic shock is controlled to avoid undesired lysis of leukocytes and platelets. The hypotonic solution is passed through the static mixer section of the instant cell lysis device for 2-30 seconds, or otherwise sufficient to lyse red blood cells, pr...
Embodiment 3
[0090] Virus clearance studies on stroma-free hemoglobin solutions
[0091] In order to demonstrate the safety of the product described in the present invention, we demonstrated the virus clearance capacity of (1) 0.65 μm diafiltration step and (2) 100 kDa ultrafiltration step through virus validation studies. This was done by intentionally adding different model viruses (encephalomyocarditis virus, pseudorabies virus, bovine viral diarrhea virus and bovine parvo vims) to these two processes in scale-down form. conduct. Four types of viruses were used in this study (see Table 3). These viruses differ in their biophysical and structural characteristics and exhibit variations in resistance to physical and chemical agents or treatments.
[0092] table 3
[0093]
[0094] The verification scheme is briefly shown in Table 4 below.
[0095] Table 4
[0096]
[0097] A summary of the results for the logarithmic (log) reduction of the four viruses in (1) 0.65 μm diafiltrati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com