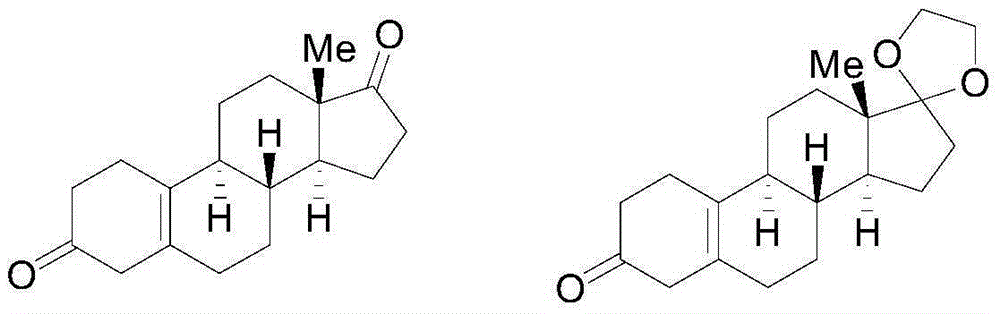
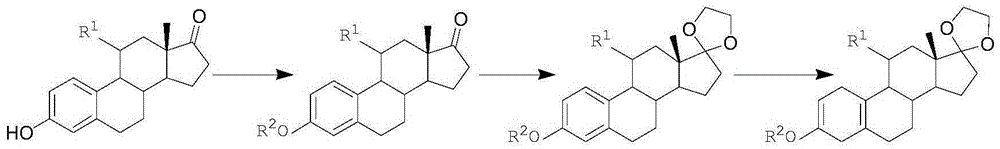
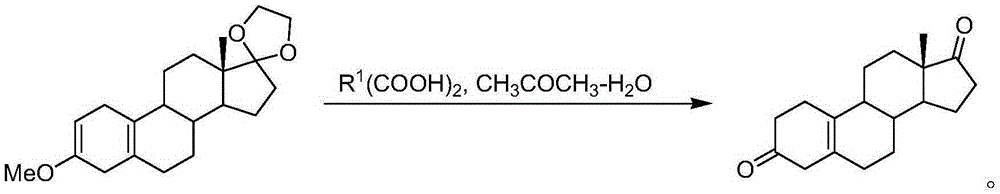Method for preparing 19-nor-5(10)-androstenone compound
A technology of androstenone and compounds, which is applied in the field of chemical preparation, can solve problems such as singleness, and achieve the effects of simple operation steps, low cost of raw materials, and high reaction conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] In the first step, add 10g of estrone and 15g of potassium carbonate into a 250ml reaction bottle, first add 100ml of solvent N,N-dimethylformamide at room temperature, then add 50ml of dimethyl carbonate, and react at 130°C for 16 hours ; After the reaction is completed, pour into ice water and stir for one hour, filter, wash with water, and dry under reduced pressure at 60°C to obtain an ether compound with a yield of 99% and an HPLC content of 98% (240nm);
[0074] In the second step, add 20ml of ethylene glycol, 25ml of triethyl orthoformate, 0.2ml of boron trifluoride ether into a dry 250ml reaction bottle, and stir at 25°C for 15 minutes; then add 10g of ether compound, 40ml of dichloromethane, React for 5 hours at ℃; TLC (thin layer chromatography) detects no raw material point, add triethylamine, stir for 10 minutes; concentrate under reduced pressure until no fraction is distilled, pour into ice water and stir for one hour, filter, wash with water, and store at ...
Embodiment 2
[0081] Preparation of 19-nor-5(10)-androstenedione: in the fourth step, add 10 g of the Birch reduction product, 160 ml of acetone, 40 ml of water, and 5 g of malonic acid into a 500 mL reaction flask, and react at 20 ° C for 5 hours. TLC detects that there is no raw material point, cool to 0 degrees, add 20% aqueous sodium hydroxide solution to adjust the pH value to neutral; recover the solvent under reduced pressure, pour it into ice water and stir for one hour, filter, wash with water, and dry under reduced pressure at 60°C to obtain 19-nor-5(10)-androstenedione, yield 95%, HPLC content 90% (202nm).
Embodiment 3
[0083] Preparation of 19-nor-5(10)-androstenedione: in the fourth step, add 10 g of the Birch reduction product, 160 ml of acetone, 40 ml of water, 20 g of malonic acid into a 500 mL reaction flask, and react at 20 ° C for 2 hours. TLC detected no raw material point, cooled to 0 degrees, added 20% sodium hydroxide aqueous solution to adjust the pH value to neutral; recovered the solvent under reduced pressure, poured into ice water and stirred for one hour, filtered, washed with water, and dried under reduced pressure at 60°C to obtain 19-nor-5(10)-androstenedione, yield 95%, HPLC content 92% (202nm).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com