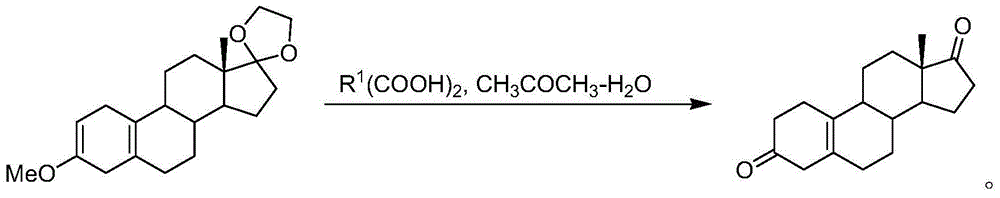Method for preparing 19-Norandrost-5(10)-ene-3,17-dione compound
A technology for androstenone and compounds is applied in the field of chemical preparation, can solve problems such as singleness, and achieves the effects of simple operation steps, cheap and easy-to-obtain raw materials, and short process routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
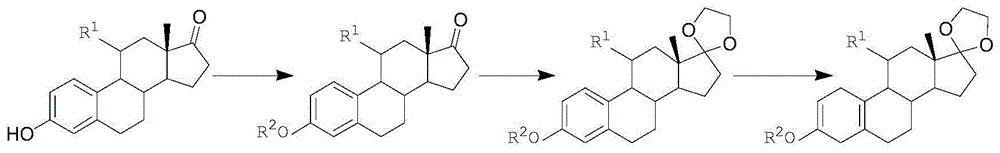
[0073] In the first step, add 10g of estrone and 15g of potassium carbonate into a 250ml reaction bottle, first add 100ml of solvent N,N-dimethylformamide at room temperature, then add 50ml of dimethyl carbonate, and react at 130°C for 16 hours ; After the reaction is completed, pour into ice water and stir for one hour, filter, wash with water, and dry under reduced pressure at 60°C to obtain an ether compound with a yield of 99% and an HPLC content of 98% (240nm);
[0074] In the second step, add 20ml of ethylene glycol, 25ml of triethyl orthoformate, 0.2ml of boron trifluoride ether into a dry 250ml reaction bottle, and stir at 25°C for 15 minutes; then add 10g of ether compound, 40ml of dichloromethane, React for 5 hours at ℃; TLC (thin layer chromatography) detects no raw material point, add triethylamine, stir for 10 minutes; concentrate under reduced pressure until no fraction is distilled, pour into ice water and stir for one hour, filter, wash with water, and store at ...
Embodiment 2
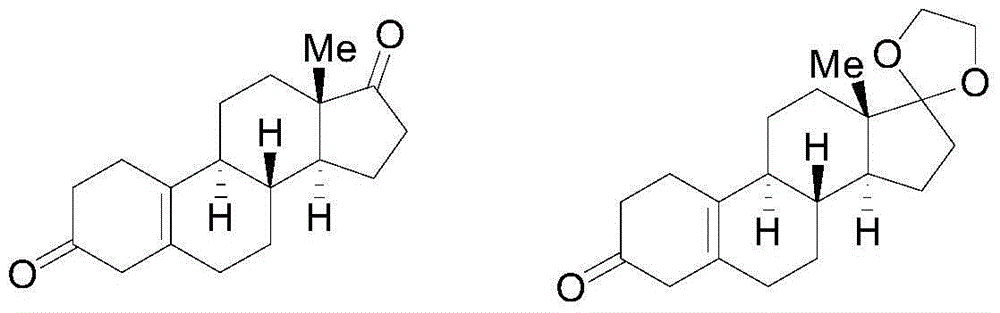
[0081] Preparation of 19-nor-5(10)-androstenedione: in the fourth step, add 10 g of the Birch reduction product, 160 ml of acetone, 40 ml of water, and 5 g of malonic acid into a 500 mL reaction flask, and react at 20 ° C for 5 hours. TLC detects that there is no raw material point, cool to 0 degrees, add 20% aqueous sodium hydroxide solution to adjust the pH value to neutral; recover the solvent under reduced pressure, pour it into ice water and stir for one hour, filter, wash with water, and dry under reduced pressure at 60°C to obtain 19-nor-5(10)-androstenedione, yield 95%, HPLC content 90% (202nm).
Embodiment 3
[0083] Preparation of 19-nor-5(10)-androstenedione: in the fourth step, add 10 g of the Birch reduction product, 160 ml of acetone, 40 ml of water, 20 g of malonic acid into a 500 mL reaction flask, and react at 20 ° C for 2 hours. TLC detected no raw material point, cooled to 0 degrees, added 20% sodium hydroxide aqueous solution to adjust the pH value to neutral; recovered the solvent under reduced pressure, poured into ice water and stirred for one hour, filtered, washed with water, and dried under reduced pressure at 60°C to obtain 19-nor-5(10)-androstenedione, yield 95%, HPLC content 92% (202nm).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com