Hepatitis B vaccine for inducing organism to generate specific immunity in state of chronic hepatitis B virus infection
A technology of hepatitis B vaccine and chronic hepatitis B, which is applied in antiviral agents, antibody medical ingredients, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1: The combination method of CL097 and aluminum adjuvant:
[0064] Preparation method 1:
[0065] Al and antigen were mixed for 30 minutes at room temperature
[0066] Mix Al and CL097 at room temperature for 30 minutes
[0067] The two are then mixed,
[0068] The free CL097 measured by mass spectrometry is 41% of the total amount added
[0069] The estimated binding capacity was 59%.
[0070] Preparation method 2:
[0071] Al and CL097 were first mixed at room temperature for 15 minutes,
[0072] Add antigen and keep at room temperature for 10 minutes,
[0073] The free CL097 of mass spectrometry is 42% of the total amount added,
[0074] The estimated binding capacity is 58%.
[0075] Preparation method 3:
[0076] Al and antigen were first mixed at room temperature for 30 minutes,
[0077] Then add CL097 at room temperature for 10 minutes,
[0078] The free CL097 measured by mass spectrometry is 44% of the total amount added,
[0079] The estimat...
Embodiment 2
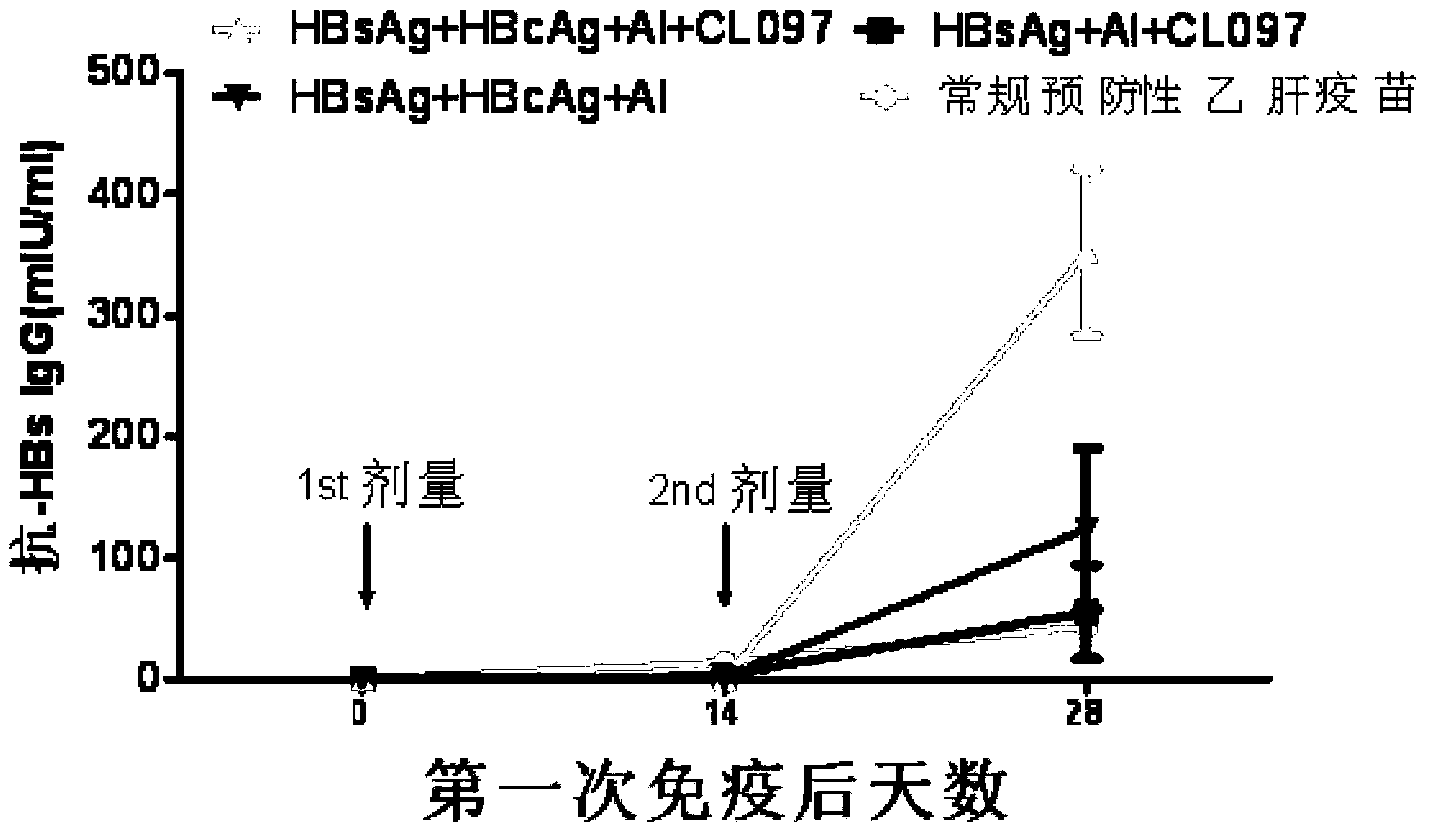
[0081] Example 2: Effects of CL097 combined with HBsAg and / or HBcAg on the humoral immune response of C57 / B6J mice
[0082] 1. Experimental animal: C57 / B6J mouse (purchased from Beijing Huafukang Biotechnology Co., Ltd., male, date of birth: March 14-21, 2012)
[0083] 2. Reagents:
[0084] 1) Hepatitis B virus surface antibody quantitative assay kit (enzyme-linked immunoassay): purchased from Beijing Wantai Biopharmaceutical Co., Ltd. (batch number: R20110905).
[0085] 2) Hepatitis B virus core antibody diagnostic kit (enzyme-linked immunoassay): purchased from Shanghai Kehua Bioengineering Co., Ltd. (batch number: 201203031). The competition method is used for detection. If the anti-HBc content in the sample to be tested is high, the absorbance value will be low, which may easily cause understanding errors. In order to avoid this situation, we use the upper limit of absorbance detection 4 to subtract the actual detection result. absorbance as its relative titer).
[0086...
Embodiment 3
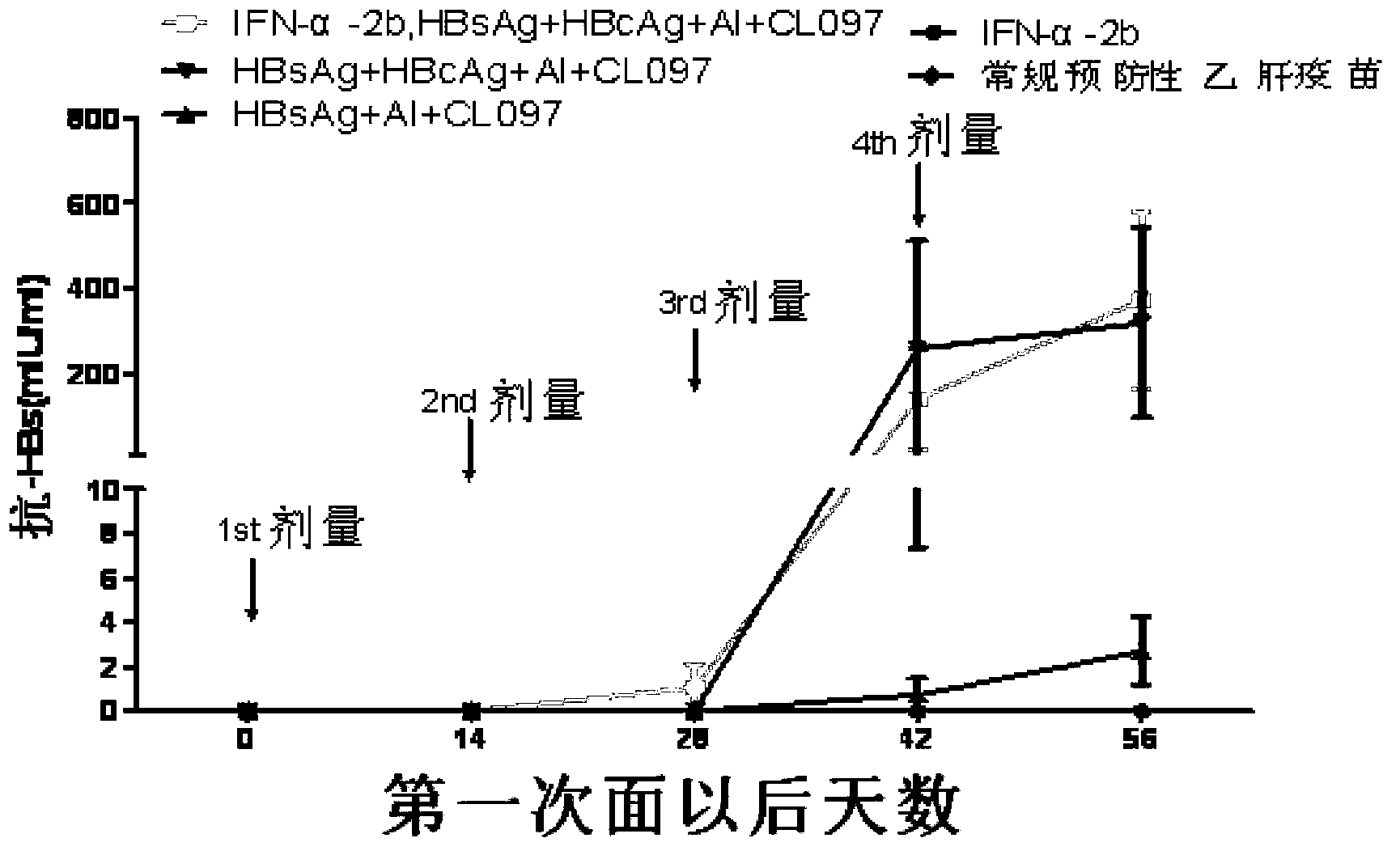
[0103] Embodiment 3: CL097 combined with HBsAg / HBcAg can break the immune tolerance of HBV transgenic mice
[0104] 1. Experimental animal: C57BL / BJ-TgN(AlblHBV)44Bri mouse, which is a kind of HBV transgenic mouse, carries the genes encoding the S, PreS1, PreS2 and HBx regions of HBV, and can be detected in serum, liver and kidney HBsAg was expressed in the mouse, but there was no virus replication (purchased from the Experimental Animal Center of Peking University Health Science Center, male, mouse birth date: September 21-28, 2011).
[0105] 2. Reagents:
[0106] 1) Hepatitis B virus surface antibody quantitative assay kit (enzyme-linked immunoassay): purchased from Beijing Wantai Biopharmaceutical Co., Ltd. (batch number: R20110905);
[0107] 2) Hepatitis B virus surface antigen diagnostic kit (enzyme-linked immunoassay): purchased from Shanghai Kehua Bioengineering Co., Ltd. (batch number: 201110031);
[0108]3) Alanine aminotransferase assay kit (rate method): purchased...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com