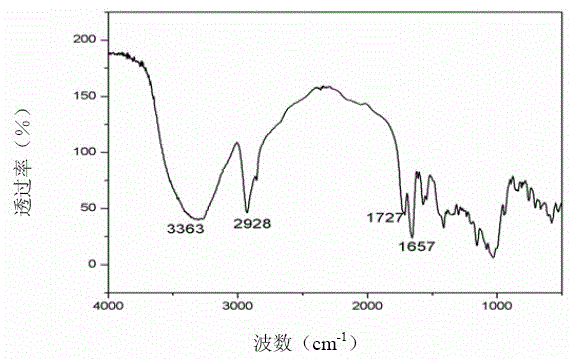
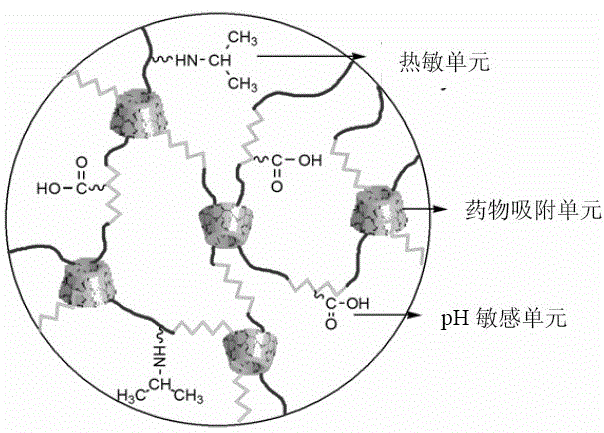
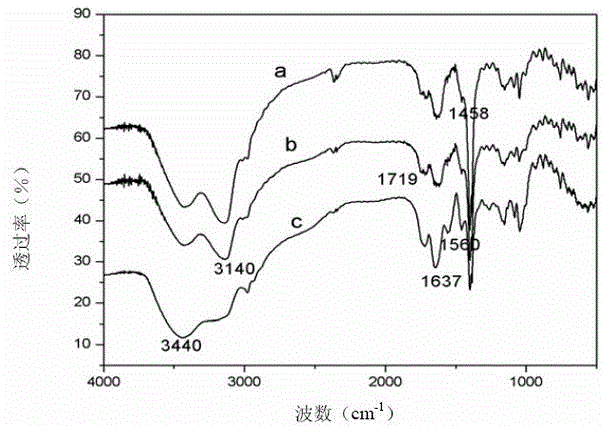
Double-stimulus response hydrogel with cyclodextrin and preparation method thereof
A dual-stimuli-response, cyclodextrin technology, applied in the direction of non-active components of polymer compounds, can solve the problems of hydrophobic drug molecule loading and effective release, and achieve the effect of easy realization and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1) Preparation of cyclodextrin modified crosslinking agent:
[0024] Weigh 1.14g of cyclodextrin and 0.1g of dimethylaminopyridine and dissolve in 10mL of dimethylformamide, then drop in 0.72g of acrylic acid, and place it in a constant temperature water bath at 60°C for stirring after it is completely dissolved. Then 2.26 g of dicyclohexylcarbodiimide was weighed and dissolved in 10 mL of dimethylformamide, and dropped into the above solution drop by drop to react for 10 h. After the reaction, it was cooled to room temperature, and the precipitated solid was removed by vacuum filtration, and the filtrate was precipitated with acetone, and the obtained white solid product was placed in a vacuum oven, and dried at 70°C until constant weight.
[0025] 2) Preparation of dual stimuli-responsive hydrogels with cyclodextrin:
[0026] Dissolve 0.24g of cyclodextrin-modified crosslinking agent, 0.24g of isopropylacrylamide and 0.12g of acrylic acid in 2mL of deionized water, a...
Embodiment 2
[0028] 1) Preparation of cyclodextrin modified crosslinking agent:
[0029] Weigh 1.14g of cyclodextrin and 0.1g of dimethylaminopyridine and dissolve in 7mL of dimethylformamide, then drop in 0.34g of acrylic acid, and place it in a constant temperature water bath at 50°C for stirring after it is completely dissolved. Then 1.07 g of dicyclohexylcarbodiimide was weighed and dissolved in 6 mL of dimethylformamide, and dropped into the above solution drop by drop to react for 8 h. After the reaction, it was cooled to room temperature, and the precipitated solid was removed by vacuum filtration. The filtrate was precipitated with acetone, and the obtained white solid product was placed in a vacuum oven and dried at 60° C. to constant weight.
[0030] 2) Preparation of dual stimuli-responsive hydrogels with cyclodextrin:
[0031] Dissolve 0.24g of cyclodextrin-modified crosslinking agent, 0.18g of isopropylacrylamide and 0.18g of acrylic acid in 2.5mL of deionized water, add 0.05g ...
Embodiment 3
[0033] 1) Preparation of cyclodextrin modified crosslinking agent:
[0034] Weigh 1.14g of cyclodextrin and 0.1g of dimethylaminopyridine and dissolve it in 18mL of dimethylformamide, then drop in 0.84g of acrylic acid, and place it in a constant temperature water bath at 50°C for stirring after it is completely dissolved. Then 2.64 g of dicyclohexylcarbodiimide was weighed and dissolved in 15 mL of dimethylformamide, and dropped into the above solution drop by drop to react for 12 h. After the reaction, cool to room temperature, remove the precipitated solid by vacuum filtration, and precipitate the filtrate with acetone. The obtained white solid product is placed in a vacuum oven and dried at 80° C. to constant weight.
[0035] 2) Preparation of dual stimuli-responsive hydrogels with cyclodextrin:
[0036] Dissolve 0.24g of cyclodextrin crosslinking agent, 0.12g of isopropylacrylamide and 0.24g of acrylic acid in 3mL of deionized water, add 0.06g of sodium bisulfite, pass n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com