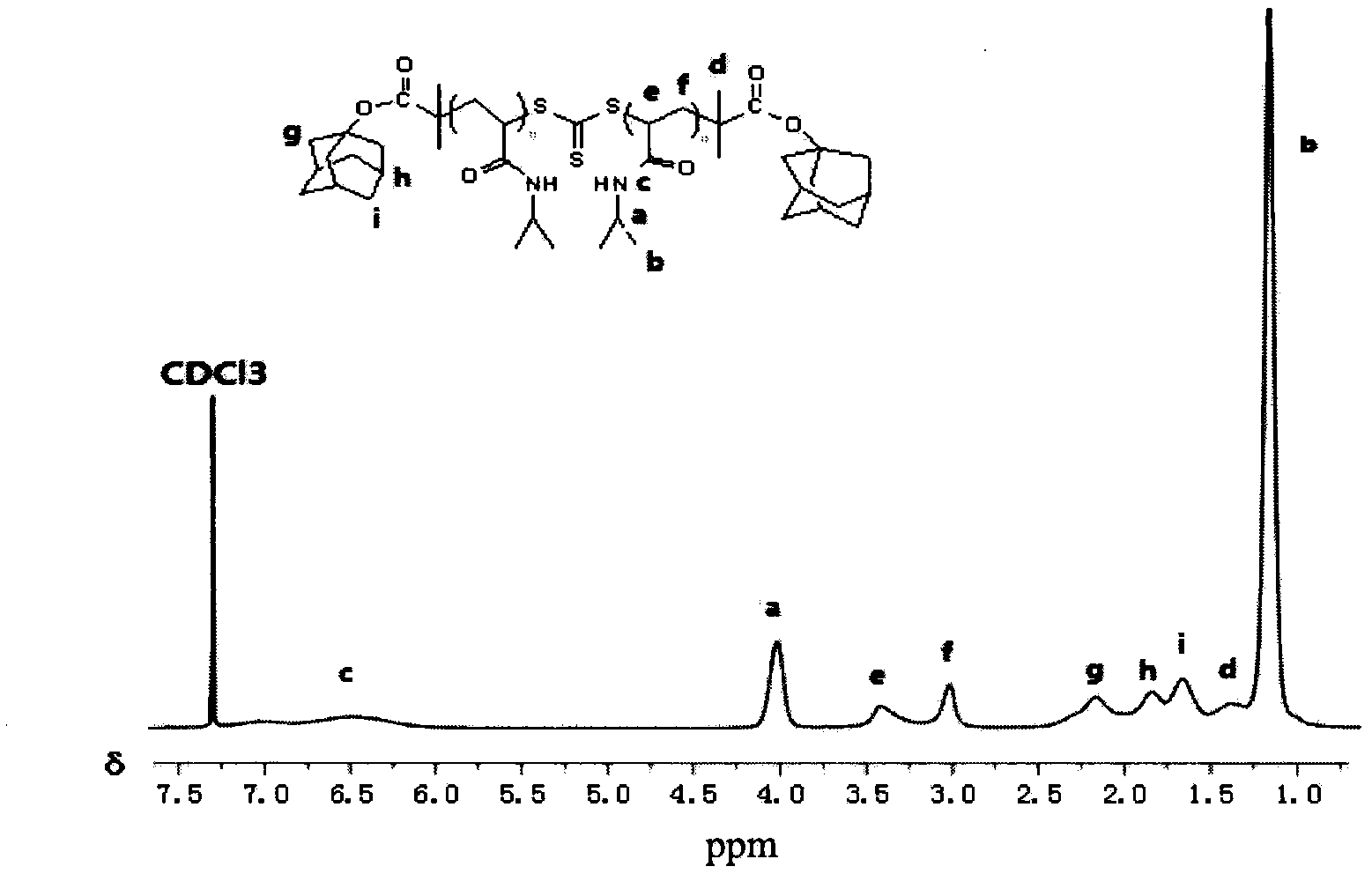
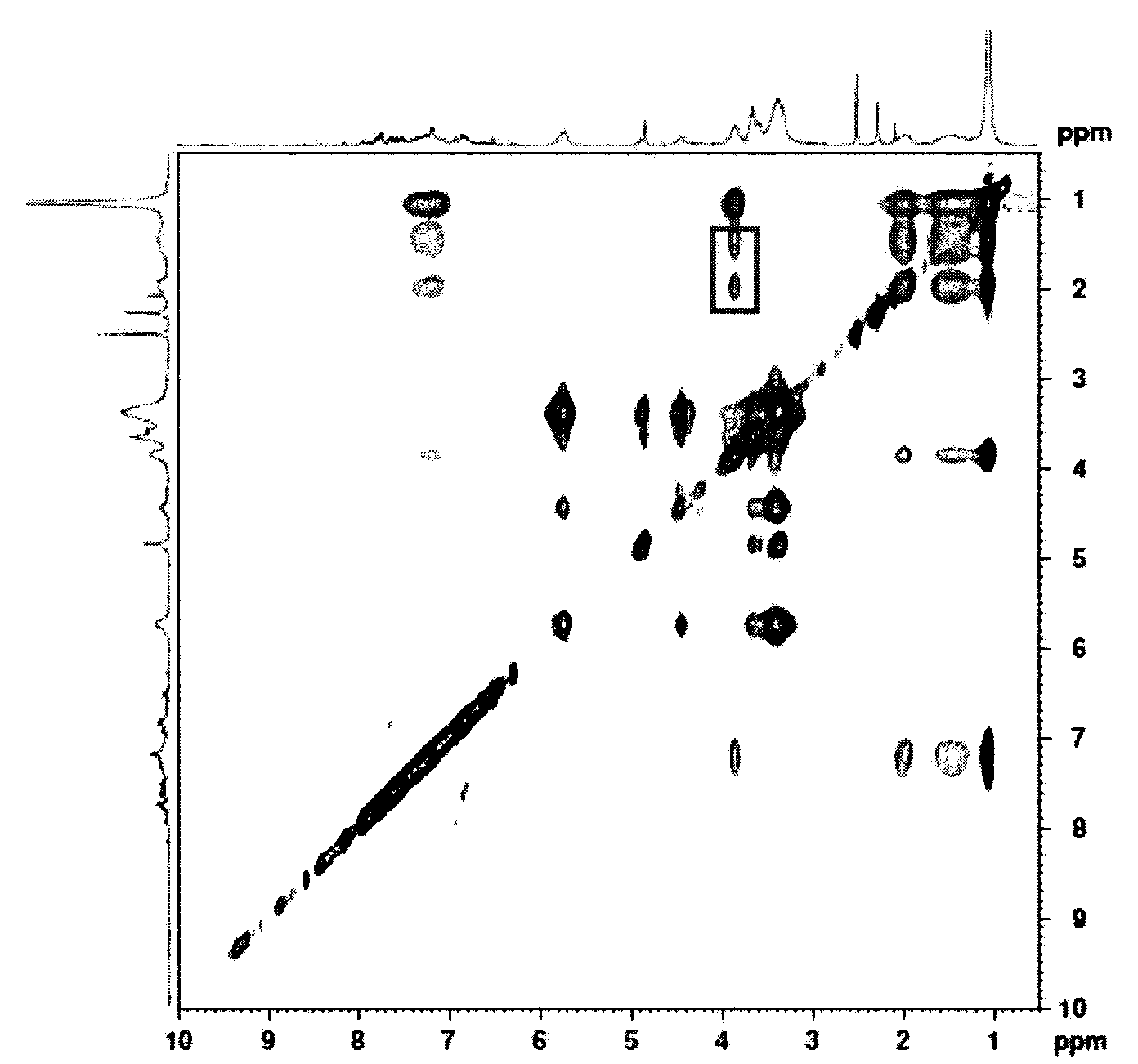
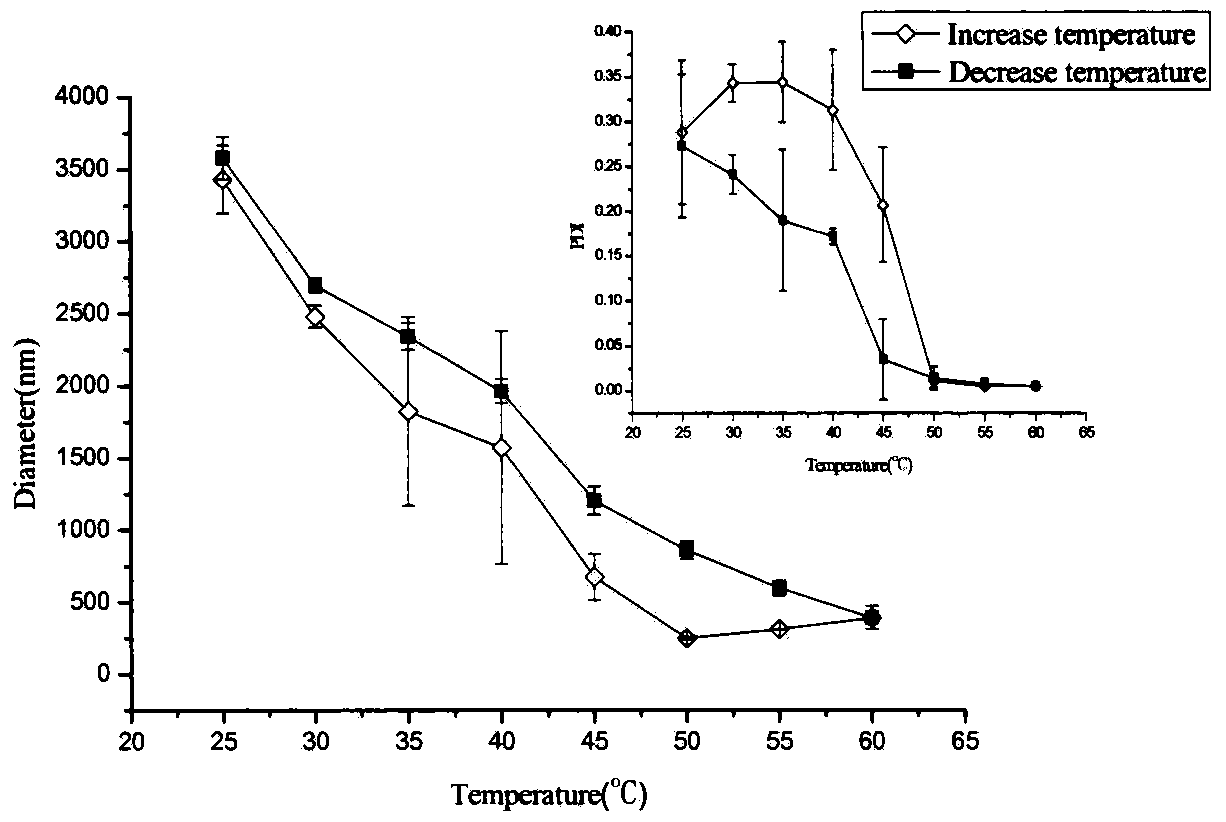
Preparation method of double-sensitivity cyclodextrin supermolecule aggregate
A supramolecular aggregate and sensitive technology, applied in the field of functional materials, can solve the problem of no photosensitive stimuli-responsive supramolecular polymer, and achieve the effect of good photosensitivity and temperature sensitivity, and broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The synthesis of embodiment 1,4-acetyl cinnamic acid
[0025] Add 4.60g (28mmol) of 4HCA and 10mL of anhydrous pyridine into a 100mL three-necked flask, install a constant temperature heating system and a stirring system, stir for 30min under a constant temperature ice bath at 0°C, then add 7.5mL of acetic anhydride at this temperature, and continue stirring After stirring for 1.5 hours, the obtained solution was heated to reflux for 5 hours under constant temperature oil bath magnetic stirring at 130°C, and the obtained solution was poured into a 500mL beaker, washed twice with 0.1mol / L hydrochloric acid solution, and filtered to obtain a white powder with a large amount of deionized water. washing. Take the filter cake and dry it under vacuum at 50°C for 24 hours to obtain cinnamic acid derivative 4ACA with acetylated hydroxy group as white powder.
Embodiment 2
[0026] The synthesis of embodiment 2,4-acetyl cinnamoyl chloride
[0027] Add 4.12g (20mmol) of 4-acetyl cinnamic acid and 20mL of thionyl chloride into a 100mL one-necked flask, then add 0.018g of DMF as a catalyst, and 10mL of dichloromethane as a solvent. ℃ under a constant temperature oil bath with magnetic stirring and heated to reflux for 7 hours, and distilled off under reduced pressure to remove the solvent and unreacted thionyl chloride. The product obtained was a pale yellow powder.
Embodiment 3、4
[0028] The preparation of embodiment 3,4HCA-CD
[0029] Add 5g (4.4mmol) of β-CD and 150mL of anhydrous pyridine into a 250mL three-neck flask, stir in a constant temperature ice bath at 0°C until β-CD is completely dissolved, and dissolve 0.7633g (3.4mmol) of 4-acetyl cinnamic acid Prepare a solution in 14 mL of anhydrous pyridine, add this solution dropwise into a three-necked flask within 20 minutes, and continue to stir and react at room temperature for 5 hours after the dropwise addition is completed. After the reaction was completed, a little water was added to terminate the reaction, and pyridine was distilled off under reduced pressure. The residue was extracted with methanol, and then recrystallized twice from methanol-water to obtain 4HCA-CD. The product obtained was a reddish-brown powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com