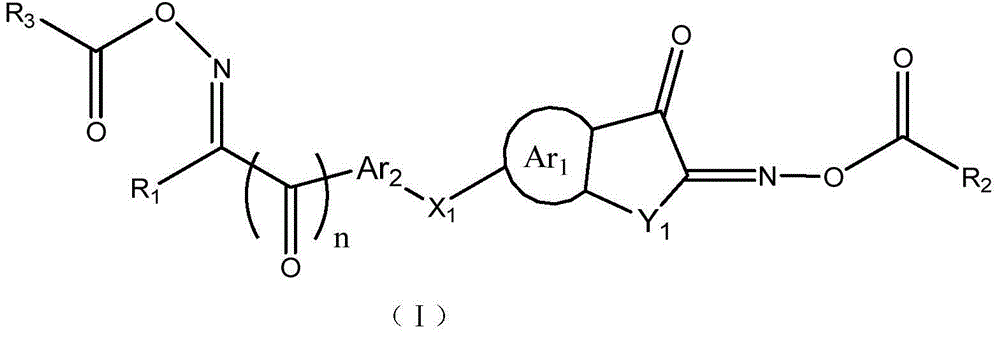
Asymmetric dioxime ester compound, making method and application thereof
A technology of compounds and general formulas, applied in applications, oxime preparation, thioether preparation, etc., can solve the problems of increased molecular weight, low activity of oxime esters, and no improvement in photosensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128] Example 1 Synthesis of 5-phenylthioindan-1-one
[0129]
[0130] Weigh 33.3g (0.2mol) of 5-chloro-1-indanone in a 50ml three-necked flask, add 30ml of N,N-dimethylformamide (DMF), 30.0g (0.27mol) of thiophenol, and 36g of anhydrous carbonic acid Potassium, nitrogen protection, stirring at 40-45°C for 6h; recover DMF under reduced pressure, add the residue to 100ml water, extract twice with 25ml 1,2-dichloroethane, combine the 1,2-dichloroethane solution, and use Wash twice with 10ml of water, spread filter paper in a Buchner funnel, use it to filter the organic phase, concentrate the filtrate to dryness, recrystallize the residue with methanol, and obtain 43.4g of light yellow crystals after drying, the yield is 90.3%, and the purity is analyzed by HPLC 98.2%. The melting range is 46.0-48.0°C.
Embodiment 2
[0131] Example 2 Synthesis of 5-(4-octanoylphenylthio)indan-1-one
[0132]
[0133] Weigh 24.04g (0.1mol) of the product 5-phenylthioindan-1-one of Example 1 and dissolve it in 120ml of 1,2-dichloroethane, cool down to 5-10°C, add 28g (0.21mol) of anhydrous Aluminum trichloride, stirring and adding 17.9g (0.11mol) octanoyl chloride dropwise, stirring for 4h after adding, treating the reaction solution with dilute hydrochloric acid, separating the organic solution, washing once with water, concentrating and recovering 1,2-dichloroethane, and The residue was recrystallized in 100ml of ethanol to obtain 33.1g of white crystals, yield 90.1%, melting range 74.3-75.2°C, 1 H-NMR data shows that the product obtained is 5-(4-octanoylphenylthio)indan-1-one; 1 H-NMR (CDCl 3 ), δ (ppm) value data: 0.8848 (t, 3H, CH 3 ), 1.2892-1.3412 (m, 8H, 4CH 2 ), 1.7359 (m,2H,CH 2 ), 2.7066 (t,2H,c-CH 2 ), 2.9526(t,2H,CH 2 ), 3.0961 (t,2H,c-CH 2 ), 7.2740 / 7.3008(d,1H,ArH), 7.3734(s,1H,ArH),...
Embodiment 3
[0134] Example 3 Synthesis of 5-(4-octanoylphenylthio)indan-1,2-dione-2-oxime
[0135]
[0136] Get 18.4g (0.05mol) of the product 5-(4-octanoylphenylthio)indan-1-one obtained in Example 2, dissolve it in a 250ml three-necked flask with 150ml ethanol, add 2g of 36% concentrated hydrochloric acid, and bathe in a water bath for 15 Stir at -20°C, add 6.2g (0.06mol) n-butyl nitrite dropwise within 15min, stir at 25°C for 5h, then cool down the reaction solution to 5-10°C, filter the precipitated yellow solid, 18.7g after drying, analyze the purity 98.90%, melting range 162.7-164.0°C, 1 H-NMR data showed that the obtained product was 5-(4-octanoylphenylthio)indan-1,2-dione-2-oxime, and the yield was 94.2%; 1 H-NMR (CDCl 3 ), δ (ppm) value data: 0.8866 (t, 3H, CH 3 ), 1.2965-1.3512 (m, 8H, 4CH 2 ), 1.7493 (m,2H,CH 2 ), 2.9737(t,2H,CH 2 ), 3.7977 (s,2H,c-CH 2 ), 7.2674 / 7.2943(d,1H,ArH), 7.3320(s,1H,ArH), 7.5281 / 7.5558(d,2H,2ArH), 7.7707 / 7.7977(d,1H,2ArH), 7.9648 / 7.9925(d, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com