Distinctive method for quantitatively detecting staphylococcal bacteria
A technique for the quantitative detection of staphylococci, which is applied in the field of specific quantitative detection of staphylococci, can solve problems such as economic losses, threats to food health, food contamination, etc., and achieve the effect of fast and simple operation and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0064] Preparation of LB liquid medium: Trypton1%, Yeast Extract0.5%, NaCl1%, pH7.0, autoclaved at 121°C for 20min;
[0065] When making LB solid plates, add 1.5% agar powder to the LB liquid medium, and autoclave at 121°C for 20 minutes;
[0066] When making LB semi-solid plates, add 0.75% agar powder to the LB liquid medium, and autoclave at 121°C for 20 minutes;
[0067] The above % means mass volume percentage, g / 100ml.
[0068] Restriction enzymes and T4 DNA ligase were purchased from NEB Company.
[0069] Nickel prepacked columns were purchased from Shanghai Sangon Bioengineering Co., Ltd.
[0070] Profile-13560 (10X) ATP microbial rapid detection system was purchased from Beijing Haozheng Zhixin Technology Co., Ltd.
[0071] The SRA reagent was purchased from Beijing Haozheng Zhixin Technology Co., Ltd., and the Profile-13560 (10X) ATP microbial rapid detection system came with it.
[0072] Luciferase was purchased from Beijing Haozheng Zhixin Technology Co., Ltd., ...
Embodiment 1
[0074] Embodiment 1, the acquisition of recombinant lysostaphin
[0075] 1. Construction of expression vector
[0076] (1) The gene sequence of Lysostaphin (Staphylococcus simulans by stashylolyticus) (Gene ID: 8864975) was obtained by BLAST on NCBI, and the gene sequence was optimized to synthesize the sequence shown in SEQ ID No.1, wherein Lysostaphin The gene of the enzyme is the DNA molecule shown in the 37th to the 777th nucleotide from the 5' end in SEQ ID No.1, and the amino acid sequence of lysostaphin is the 13th from the N-terminus in SEQ ID No.2 from position 258 to amino acid 258. The optimized sequence makes it easier for lysostaphin to be soluble expressed in the Escherichia coli prokaryotic expression system.
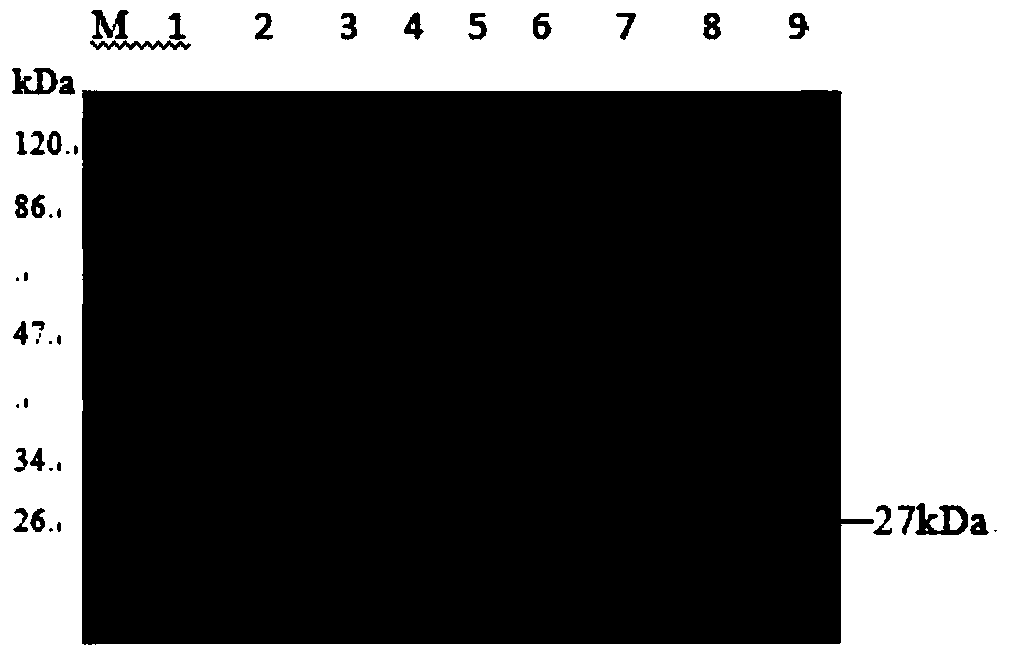
[0077] (2) Digest the DNA molecule shown in SEQ ID No.1 with BamHI and HindIII to obtain a gene fragment; use BamHI and HindIII to double digest the expression vector pQE30 to obtain a large fragment of the vector; connect the gene fragment to the large...
Embodiment 2
[0106] Embodiment 2, drop method detects recombinant lysostaphin bactericidal activity in vitro
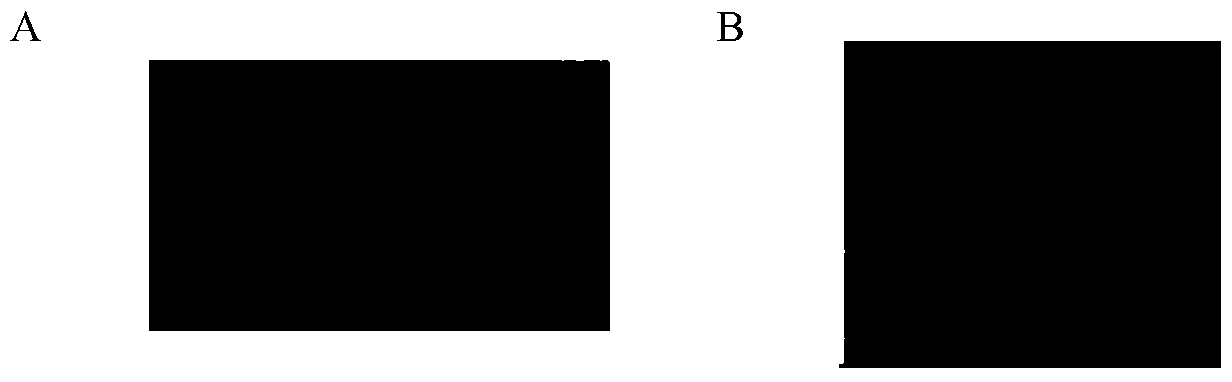
[0107] One strain of Staphylococcus aureus and 173 strains of clinically isolated Staphylococcus aureus were cultured to the logarithmic phase, and 500ul each was taken to spread double-layer LB plates, and stood at room temperature for 10min. Pipette 1ul of recombinant lysostaphin droplet on the plate, and use 0.01mol / L PBS with pH7.2 as blank control, and each group has three parallels. Incubate the plate upside down at 37°C for 3-4 hours, observe the results, and the results are as follows image 3 shown.
[0108] image 3 A is the recombinant lysostaphin group, image 3 B is the PBS (blank control) group.
[0109] image 3 It showed that there were clear circular lysis spots in the area dripped with recombinant lysostaphin, but there was no lysis spots in the PBS (blank control) group, indicating that the recombinant lysostaphin had bactericidal activity against Staphyloc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com