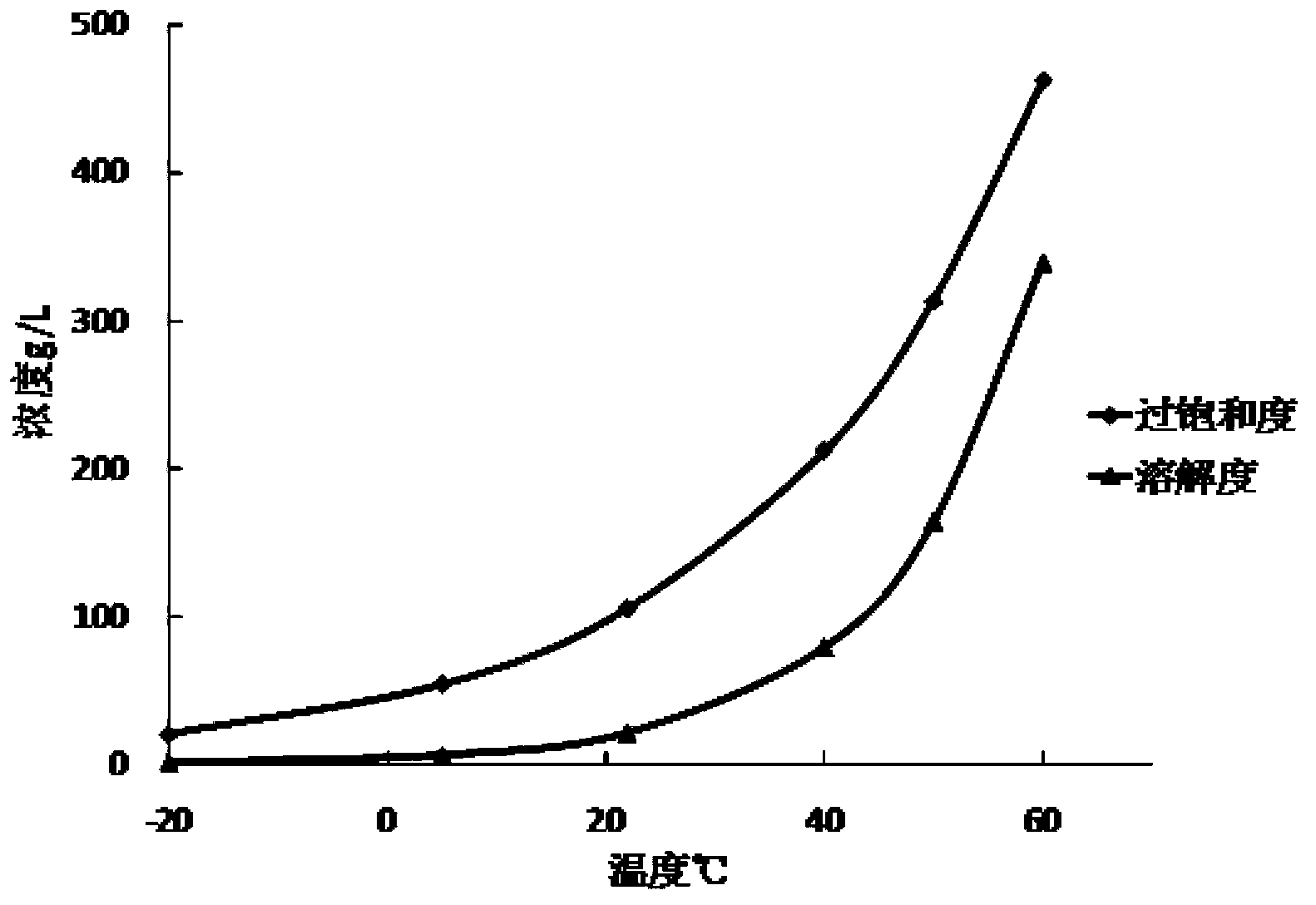
Preparation method of micro-powder capecitabine
A technology of capecitabine and micropowder, which is applied in the preparation of micropowder capecitabine and the field of capecitabine preparation, which can solve the problem that the particle size of capecitabine is not easy to control and large particle size is not good for capecitabine Dissolution of bine, crystal form change of capecitabine, etc., to achieve clinical drug safety, facilitate granulation of preparations, and ensure quality uniformity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Add 20kg of crude capecitabine and 100L of ethyl acetate to a 200L reactor, heat up to 70°C to dissolve; cool down to 50°C, add 2kg of capecitabine seed crystals (particle size d(0.1)≥1μm, d(0.9) ≤30μm), keep stirring for 2 hours until a large number of crystals precipitate, cool to 0°C, centrifuge, and dry to obtain 20.4kg of micropowdered capecitabine, with a yield of 92.7%, a purity of 99.92%, and a melting point of 117-119°C.
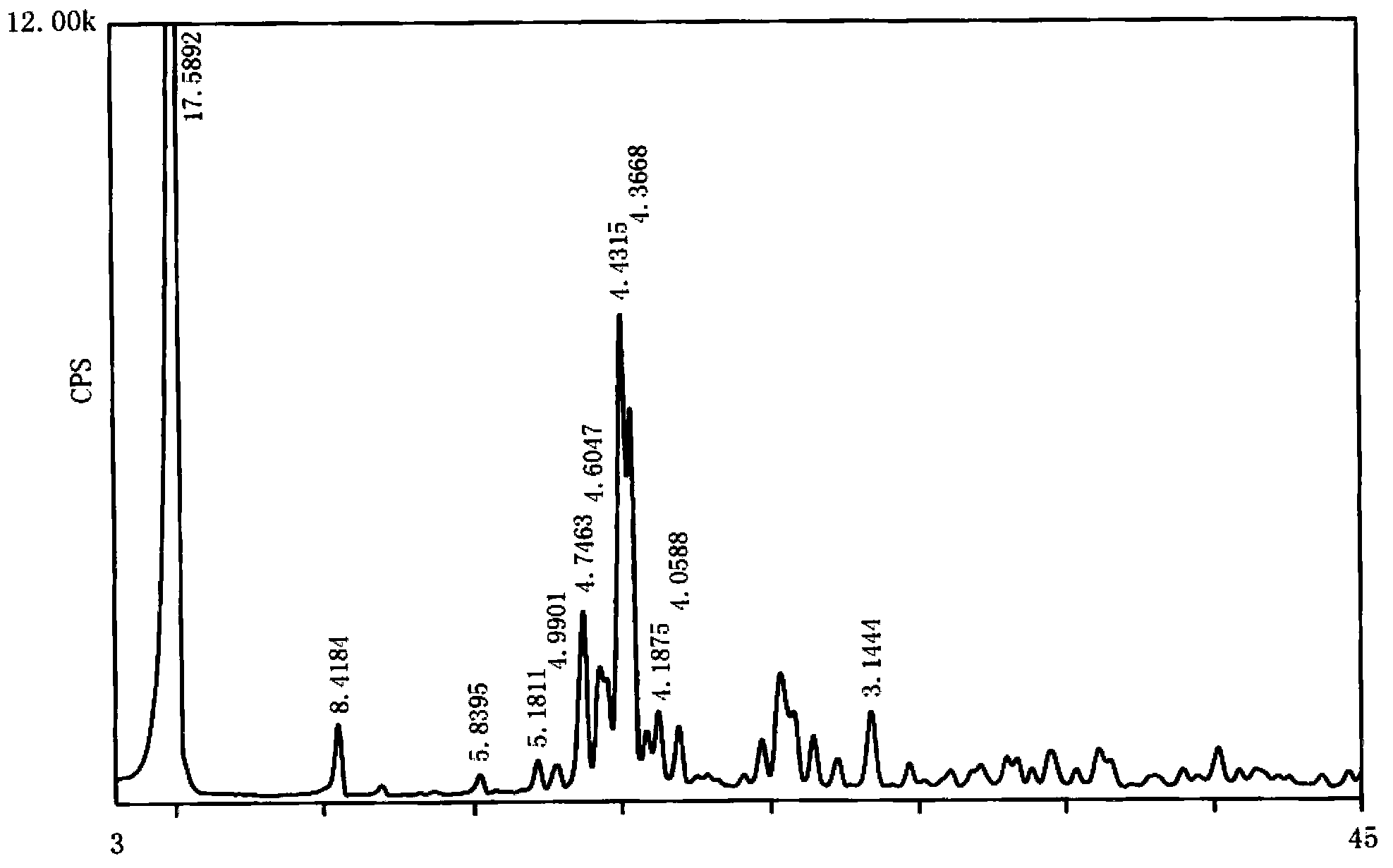
[0054] Gained micropowder type capecitabine is measured through X-ray powder diffraction pattern, uses Cu-Kα radiation, and the X-ray powder diffraction expressed in 2θ angle is at 5.0°±0.2°, 10.5°±0.2°, 15.2°±0.2° °, 17.1°±0.2°, 17.8°±0.2°, 18.7°±0.2°, 19.3°±0.2°, 20.0°±0.2°, 20.3°±0.2°, 21.2°±0.2°, 21.9°±0.2°, 28.4°±0.2° has a characteristic peak, such as figure 2 shown.
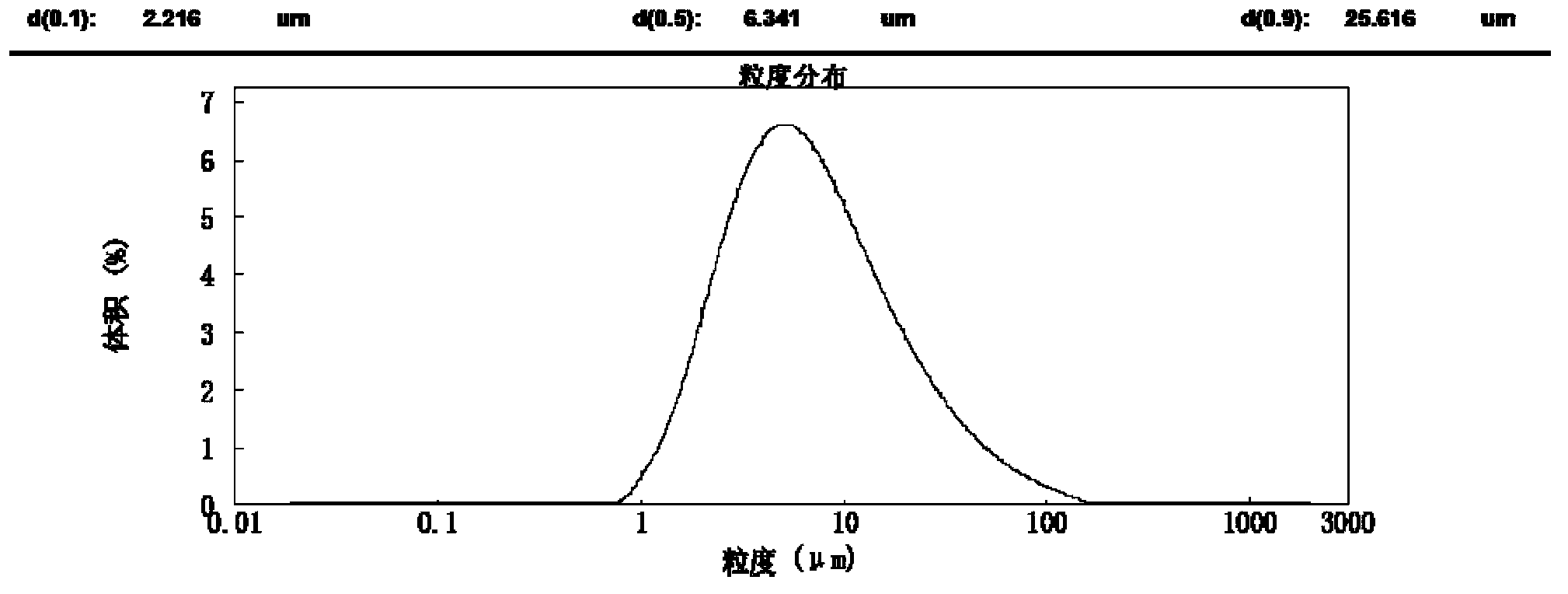
[0055] The obtained powder is measured by a laser particle size analyzer, and its particle size range is d (0.1): 2.216 μm, d (0.5): 6.341 μm, d (0.9): 25.616 μm, and ...
Embodiment 2
[0061] Add 200g capecitabine crude product and 2L ethyl acetate to a 3L flask, heat up to 60°C to dissolve; cool down to 40°C, add 4g capecitabine seed crystals (particle size d(0.1)≥1μm, d(0.9)≤ 30 μm), kept stirring for 6 hours until a large number of crystals were precipitated, cooled to -5°C, filtered with suction, and dried to obtain 185g of micropowdered capecitabine with a yield of 90.7%, a purity of 99.94%, and a melting point of 118-120°C.
[0062] The obtained powder is measured by a laser particle size analyzer, and its particle size range is d (0.1): 2.296 μm, d (0.5): 5.859 μm, d (0.9): 18.119 μm, and the results are as follows Figure 4 shown.
Embodiment 3
[0080] As described in Example 1, the difference is that the capecitabine seed crystal particle size is d(0.1) ≥ 1 μm, d(0.9) ≤ 20 μm; the obtained micronized capecitabine particle size is d(0.1) ≥ 1 μm, d(0.9)≤30μm. The obtained micropowder type capecitabine has a purity of 99.93%, determined by X-ray powder diffraction pattern, using Cu-Kα radiation, and the X-ray powder diffraction represented by 2θ angle is at 5.0°±0.2°, 10.5°±0.2°, 15.2°±0.2°, 17.1°±0.2°, 17.8°±0.2°, 18.7°±0.2°, 19.3°±0.2°, 20.0°±0.2°, 20.3°±0.2°, 21.2°±0.2°, 21.9° ±0.2°, 28.4°±0.2° have characteristic peaks.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com