Method for preparing formaldehyde and ozone removal catalyst at room temperature
A technology of catalyst and oxidant, applied in the field of preparation of catalyst MnO2, which can solve the problems of high price and limitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
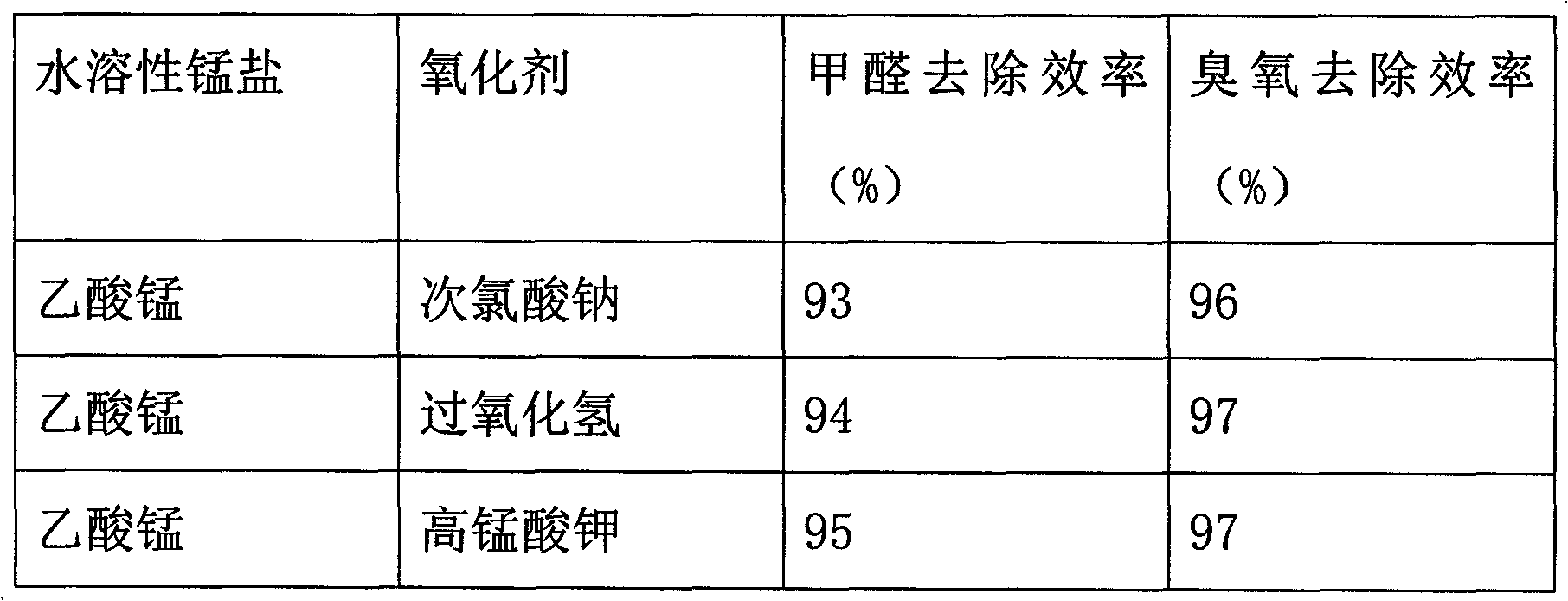
[0018] Add 1000 g of water to a 5 L glass, add manganese acetate (294 g) and stir to dissolve it. Add 2000g of water to another 5L glass, add potassium permanganate (126g) and stir to dissolve it. Use a dropping funnel to add the potassium permanganate solution dropwise to the manganese acetate solution being stirred, and control the dropping rate to 300g / min. After 15 minutes of dropping, control the dropping rate to 450g / min until the dropping is completed. After continuing to stir for 2h, stop stirring, and age for 8h. Filter the obtained precipitate, wash it with deionized water three times, and suction filter to obtain the precipitate, dry it at 105°C, and roast it at 200°C for 2 hours to obtain the MnO2 catalyst.
[0019] The BET specific surface area of the MnO2 catalyst is 180m 2 / g.
[0020] The performance test of the catalyst for removing formaldehyde was carried out in a continuous-flow fixed-bed reactor. Load 1g of the catalyst in a glass tube, at room tempe...
Embodiment 2
[0023] Add 1000 g of water to a 5 L glass, add manganese acetate (294 g) and stir to dissolve it. Add 2000g of water to another 5L glass, add potassium permanganate (126g) and stir to dissolve it. Use a dropping funnel to add the potassium permanganate solution dropwise to the manganese acetate solution being stirred, and control the dropping rate to 300 g / min until the dropping is completed. After continuing to stir for 2h, stop stirring, and age for 8h. Filter the obtained precipitate, wash it with deionized water three times, and suction filter to obtain the precipitate, dry it at 105°C, and roast it at 200°C for 2 hours to obtain the MnO2 catalyst.
[0024] The BET specific surface area of the MnO2 catalyst is 350m 2 / g.
[0025] The detection method of the removal efficiency of formaldehyde and ozone is the same as that in Example 1, the removal efficiency of formaldehyde is 96%, and the removal efficiency of ozone is 99%.
Embodiment 3
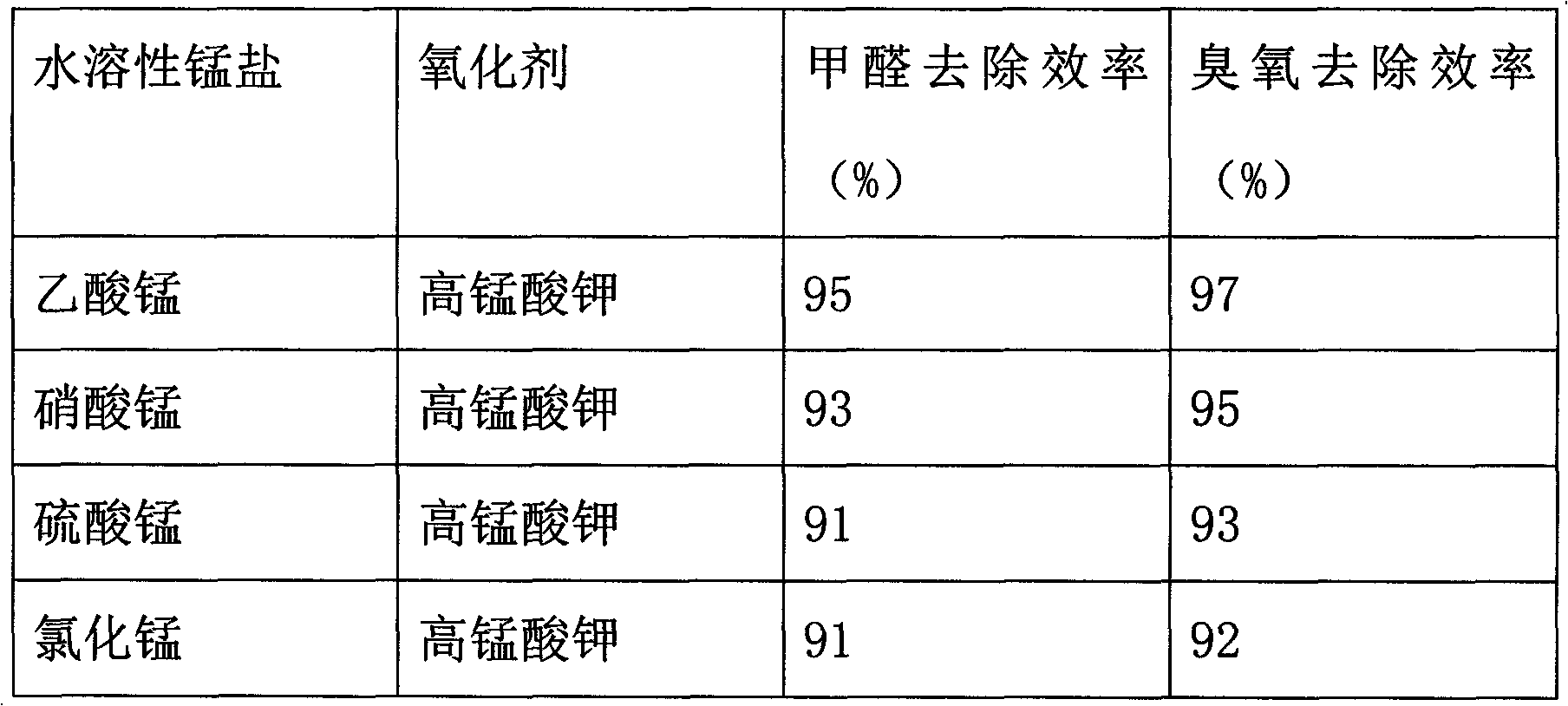
[0027] The preparation method of the catalyst in this embodiment is basically the same as that in Embodiment 1, the difference is that in this embodiment, the soluble manganese salt is replaced with one of manganese nitrate, manganese sulfate or manganese chloride, and the results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com