Modified hyaluronic acid based macromolecule lipidosome and preparation method thereof
A hyaluronic acid and polymer technology, applied in the pharmaceutical field, can solve the problems of easy leakage of encapsulated drugs, poor biocompatibility, poor physical and chemical stability, etc., and achieve the effects of short preparation cycle, good stability and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
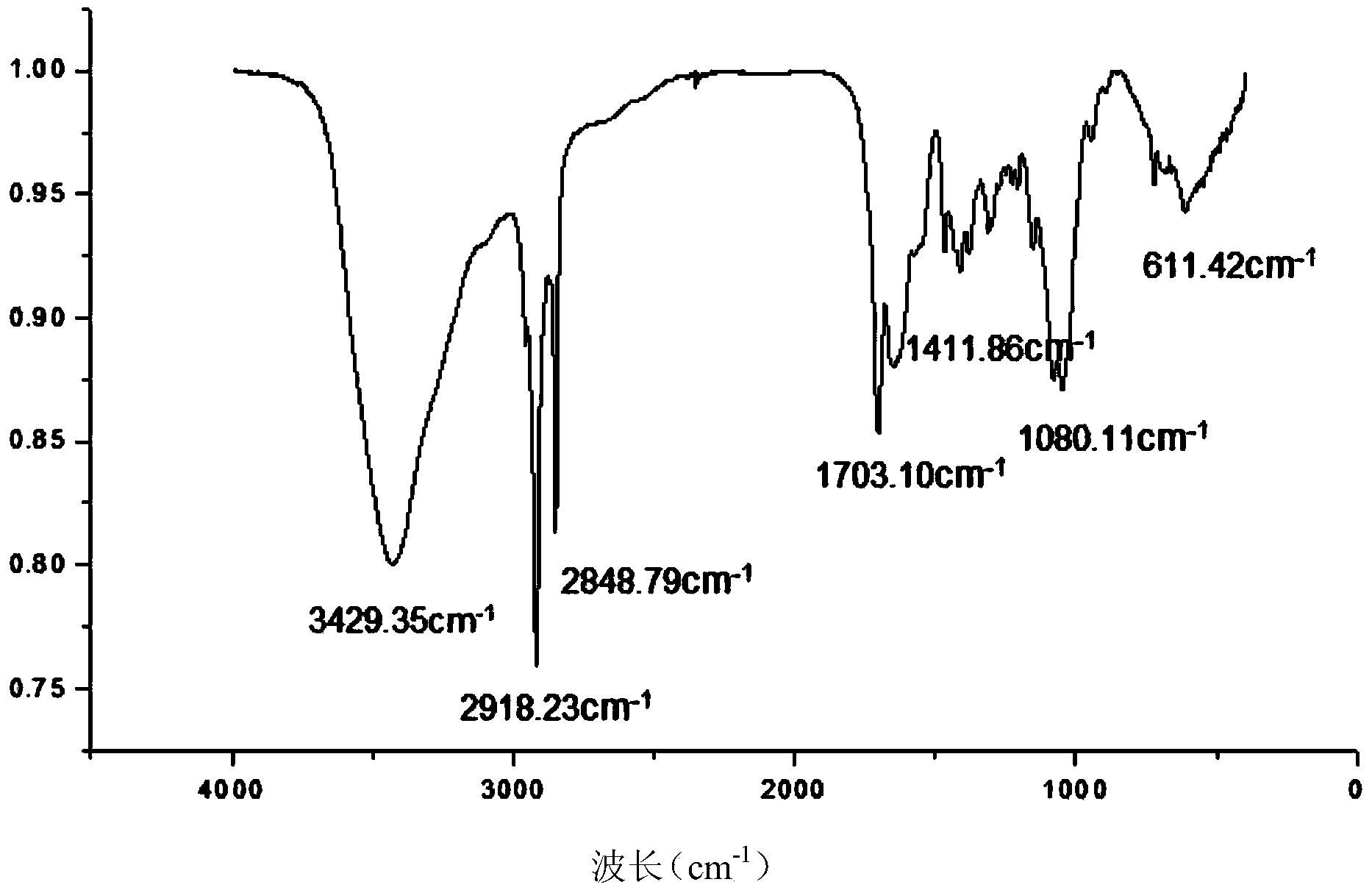
[0029] Synthesis of stearic acid-modified hyaluronic acid. Take 1 g of hyaluronic acid with a molecular weight of 10,000 and 5 g of stearic acid into a beaker, add 12 mL of water, 36 ml of dimethyl sulfoxide, 1 g of EDC and 0.05 g of DMAP into the flask, stir magnetically, and react for 3 hours. After the reaction, put the product into a dialysis bag with a molecular weight of 8000-14000 for dialysis for 3 days, and freeze-dry to obtain stearic acid-modified hyaluronic acid. Such as figure 1 Infrared analysis test results show that 3430cm -1 The broad peaks on the left and right are multiple absorption peaks formed by the overlapping of stretching vibrations of -OH and -COOH in the hyaluronic acid structure, 2918, 2848cm -1 for—CH 3 , and—CH 2 The stretching vibration absorption peak of , 1080.11cm -1 The peak is the stretching vibration absorption peak of —C—O—C— in the ester bond, and the 1410cm -1 left and right - CH 3 and—CH 2 The appearance of new absorption peak...
Embodiment 2
[0031] Synthesis of stearic acid-modified hyaluronic acid. Take 5g of hyaluronic acid with a molecular weight of 50,000, and add 1g of stearic acid into a beaker, add 200mL of water, 100ml of dimethyl sulfoxide, and 1g of EDC and 0.5g of DMAP into the flask, stir magnetically, and react for 12 hours. After the reaction, put the product into a dialysis bag with a molecular weight of 8000-14000 for dialysis for 10 days, and freeze-dry to obtain stearic acid-modified hyaluronic acid.
Embodiment 3
[0033] Synthesis of stearic acid-modified hyaluronic acid. Take 1 g of hyaluronic acid with a molecular weight of 20,000 and 1 g of stearic acid into a beaker, add 10 mL of water, 10 mL of dimethyl sulfoxide, and 0.5 g of EDC and 0.2 g of DMAP into the flask, stir magnetically, and react for 6 hours. After the reaction, put the product into a dialysis bag with a molecular weight of 8,000-14,000 for dialysis for 5 days, and freeze-dry to obtain stearic acid-modified hyaluronic acid.
PUM
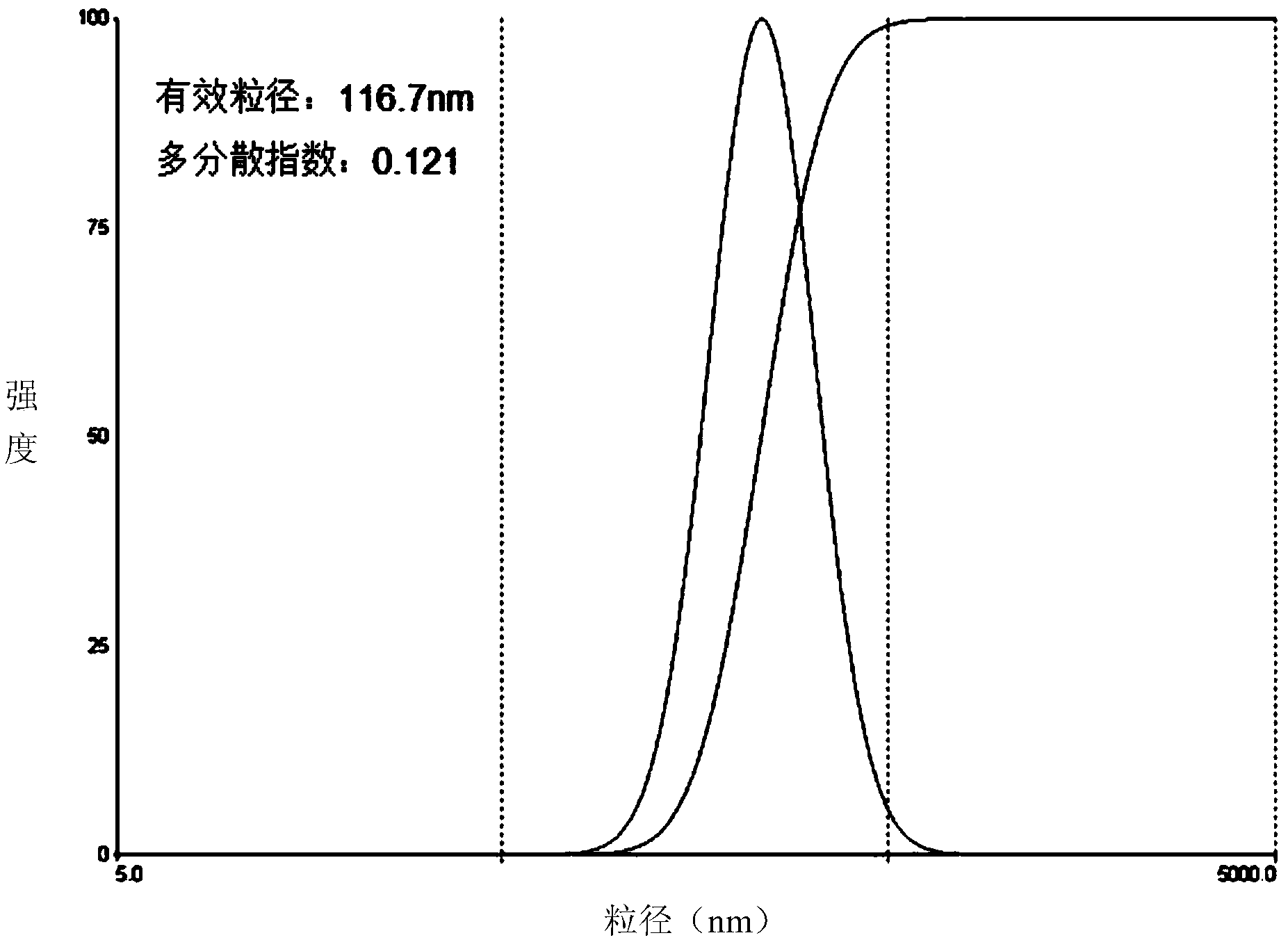
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com