Environment-friendly high-efficiency method for synthesizing adipic acid by catalytically oxidating adipic dialdehyde
A catalytic oxidation, environment-friendly technology, applied in the direction of chemical instruments and methods, preparation of organic compounds, physical/chemical process catalysts, etc., can solve the problems of environmental pollution, high price, high cost, etc., and achieve an environmentally friendly catalytic conversion process, significantly Industrial application value, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Example 1: h 2 WO 4 -1 # catalyst
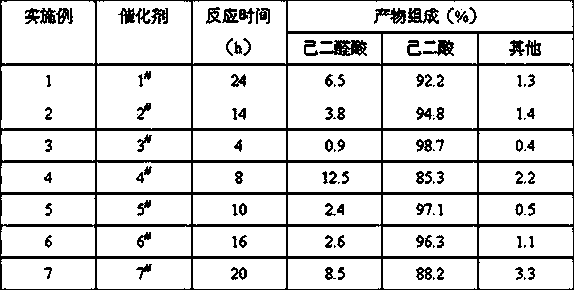
[0013] In a 25 ml three-neck round bottom flask equipped with stirring, reflux condensation and a thermometer, add 0.25 mmol tungstic acid H 2 WO 4 and 0.02 mol of hydrogen peroxide at a concentration of 30%, and stirred at room temperature for 50 minutes. After adding 0.01 mole of adipaldehyde solution dropwise, the stirring speed was increased. By heating in a water bath, the temperature of the bath was controlled to rise from 65°C to 95°C in about 2 hours, and the reflux of the reactant was maintained. After the reaction is completed, the analysis results are shown in Table 1.
Embodiment 2
[0014] Example 2: h 3 [P(W 3 o 10 ) 4 ]·10H 2 O-2 # catalyst
[0015] In a 25ml three-neck round bottom flask equipped with stirring, reflux condensation and thermometer, add 0.02mmol of phosphotungstic acid and 0.022mol of hydrogen peroxide with a concentration of 10%, respectively, and stir at room temperature for 30 minutes. After adding 0.01 mole of adipaldehyde solution dropwise, the stirring speed was increased. By heating in a water bath, the temperature of the bath was controlled to rise from 40°C to 90°C in about 3 hours, and the reflux of the reactants was maintained. After the reaction is completed, the analysis results are shown in Table 1.
Embodiment 3
[0016] Example 3: 20% WO 3 / SnO 2 -3 # catalyst
[0017] In a 25 ml three-neck round bottom flask equipped with stirring, reflux condensation and a thermometer, add 20% WO with a tungsten content of 1% relative to the molar content of adipaldehyde 3 / SnO 2 And after 0.02 mol of hydrogen peroxide with a concentration of 50%, stir at room temperature. After adding 0.01 mole of adipaldehyde solution dropwise, the stirring speed was increased. By heating in a water bath, the temperature of the bath was controlled to rise from 60°C to 92°C in about 2 hours, and the reflux of the reactants was maintained. After the reaction is completed, the analysis results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com