Mixing stem cell injection and preparation method thereof
A technology of mixing stem cells and injections, which is applied in the direction of anti-toxins, drug combinations, and pharmaceutical formulas, can solve the problems of umbilical cord blood hematopoietic stem cells restricting the scope of clinical application, and achieve the goal of promoting proliferation and maintaining stem cell characteristics, good effect, and inhibiting agglutination. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Umbilical cord blood and umbilical cord samples were obtained from healthy pregnant women and infants. Hepatitis B, hepatitis C, syphilis, AIDS, cytomegalovirus, TORCH, mycoplasma, chlamydia, G-6PD and thalassemia were all negative. After the specimens were collected, they were transported back to the blood bank to maintain the transport conditions at 4-8°C. Prepare mixed stem cell injections as follows.
[0039] 1. Preparation of Umbilical Cord Mesenchymal Stem Cells
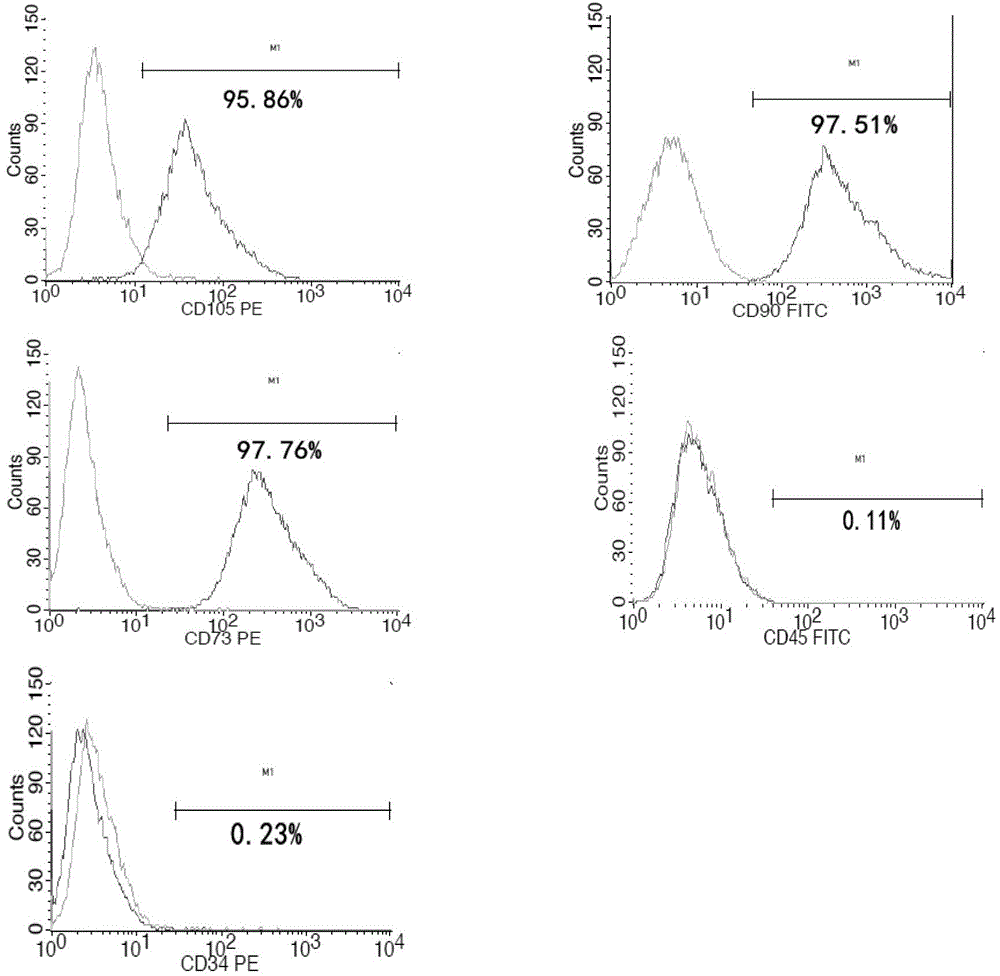
[0040] Mesenchymal stem cells are extracted from the umbilical cord tissue of healthy people, and the obtained mesenchymal stem cells are cultured and expanded in vitro. After the primary cells are collected, they are subcultured at a ratio of 1:3. When the cells cover about 80% of the density of the bottom of the bottle, they are digested and collected with trypsin, and subcultured at the same ratio, up to the third generation. Collect the cells of the 3rd generation. At this time, the collected cell...
Embodiment 2
[0073] With reference to the method of embodiment 1, obtain about 1 * 10 7 ~5×10 7 Hematopoietic stem / progenitor cells and 2×10 7 mesenchymal stem cells. The prepared hematopoietic stem / progenitor cells and mesenchymal stem cells were added to normal saline containing 5% AB umbilical cord plasma to make the concentration of the two kinds of stem cells respectively 5×10 5 / ml and 2×10 5 / ml, prepared as mixed stem cell injection.
Embodiment 3
[0075] The mixed stem cell injection prepared in Example 2 can be mixed with umbilical cord blood mononuclear cells separated by conventional methods. The number of cord blood mononuclear cells should not be less than 5×10 8 indivual.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com