Glutathione fluorescence probe as well as preparation method and application thereof
A fluorescent probe, glutathione technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of time-consuming and cumbersome process of invertase preparation, and achieve easy large-scale production and detection sensitivity. High, selective and specific effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of Glutathione Fluorescent Probe
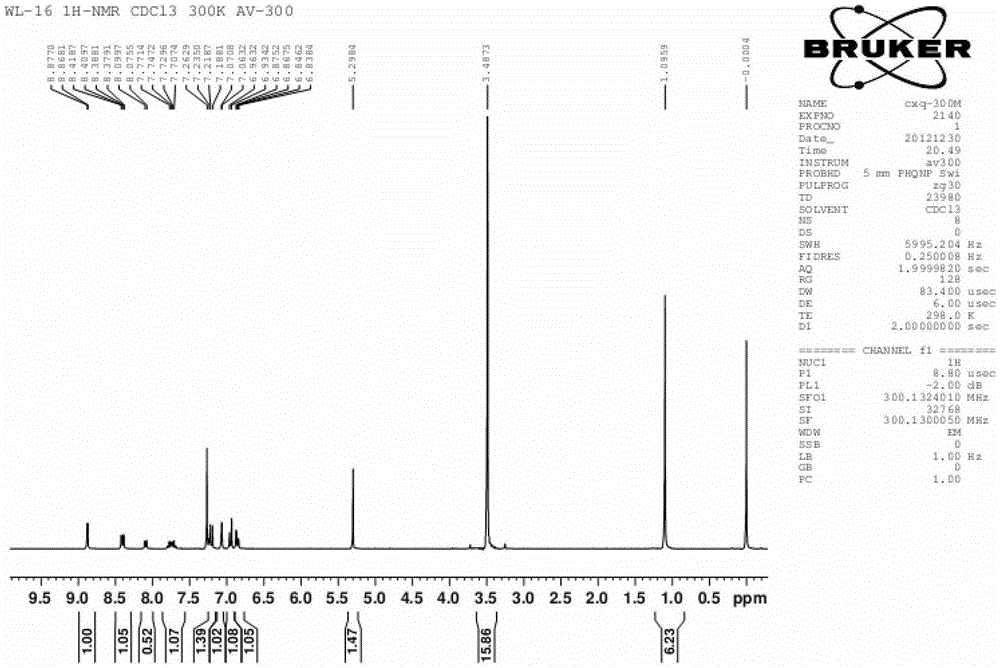
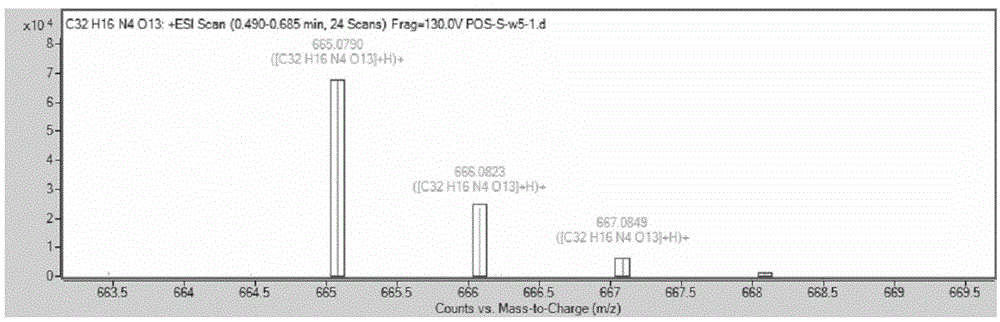
[0033] Dissolve 1.861g of 2,4-dinitrofluorobenzene, 1.994g of fluorescein and 2.764g of potassium carbonate in 20mL of anhydrous DMF; under nitrogen protection, react at 75°C for 2 hours; after the reaction, add 20mL of distilled water to wash , filtered, and the obtained solid was dried in a vacuum oven to obtain a crude product; using dichloromethane as an eluent, the crude product was purified on a silica gel column to obtain 2,4-dinitrophenyl fluorescein ether as a yellow solid 1.214g, which is the pure glutathione fluorescent probe ( 1 H-NMR chart and high-resolution mass spectrogram see figure 1 , figure 2 ). The measured molecular weight of the obtained pure fluorescent probe is 664.
[0034] Present embodiment process route:
[0035]
Embodiment 2
[0036] Example 2 The spectral properties of the prepared glutathione fluorescent probe reacting with various amino acids
[0037] Weigh 6.6 mg of the glutathione fluorescent probe prepared in Example 1, and prepare a 10 mL acetonitrile solution with a concentration of 1 mM as the mother solution.
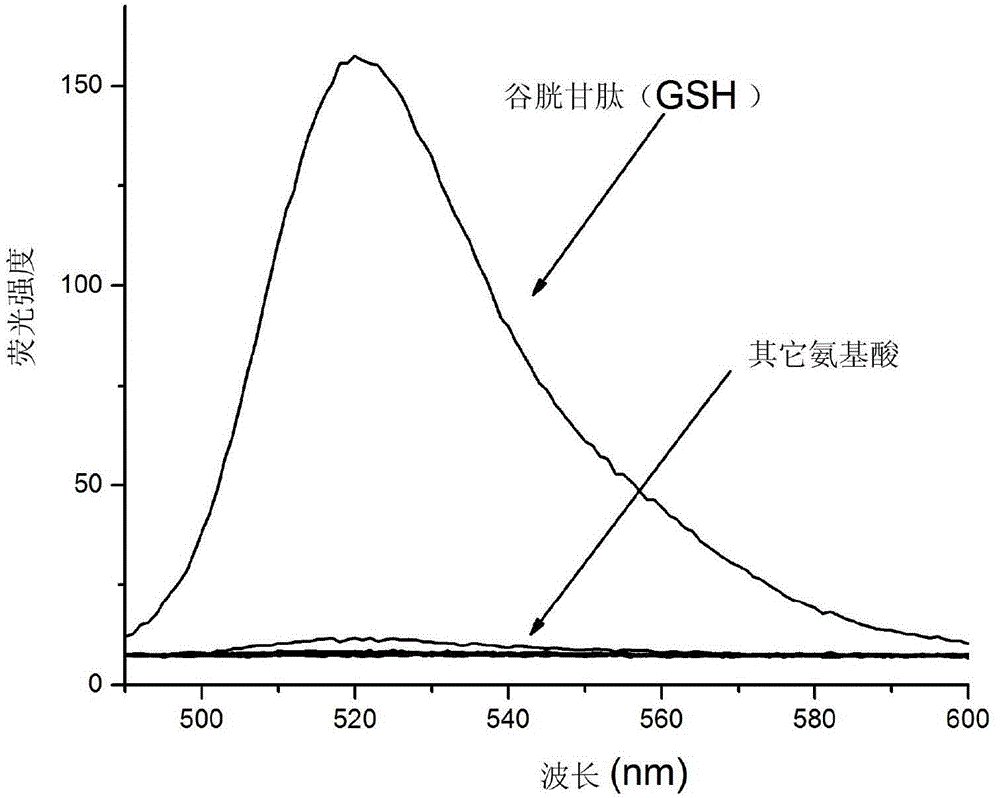
[0038] Fluorescence spectrum test: Add 15 μL of the above mother solution and 1.5 mM cetyltrimethylamine bromide to a certain amount of 20 mM ethanol-PBS buffer solution (3:7v / v, pH7.4), and then add various amino acids: Glutathione, cysteine, homocysteine, glycine, phenylalanine, methionine, glutamic acid, glutamine, lysine, arginine, histidine, alanine One of acid, serine and tyrosine, so that the final concentration of amino acid is 50 μM, and the final concentration of fluorescent probe is 5 μM. After reacting at room temperature for 30 minutes, the fluorescence emission spectrum was measured at the excitation light wavelength of 485nm. The slit width for excitation and emissi...
Embodiment 3
[0043] Example 3 Spectral Properties of Glutathione Fluorescent Probe and Glutathione Reaction Product
[0044] Add 3 μL of the mother solution in Example 2 and 1.5mM CTAB to a certain amount of 20mM ethanol-PBS buffer solution (3:7v / v, pH7.4), and then add different equivalents of glutathione solution to make the fluorescent probe The final concentration of needles was 1 μM, and the final concentrations of glutathione were 0 μM, 1 μM, 2 μM, 3 μM, 4 μM, 5 μM, 6 μM, 7 μM, 8 μM, 9 μM, and 10 μM, respectively. After reacting at room temperature for 30 minutes, measure its fluorescence emission spectrum. The fluorescence emission spectrum is excited at 485nm; the slit width of excitation and emission is 3nm. The resulting fluorescence increment graph is shown in Figure 5 ; Make a working curve with the fluorescence intensity data at 520nm, the results are shown in Image 6 .
[0045] The experimental results show that the fluorescence intensity after the reaction increases with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com