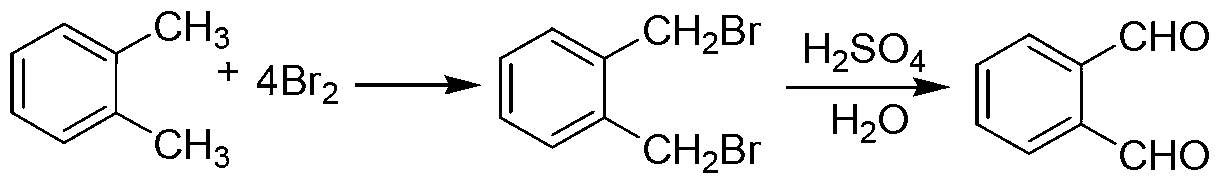
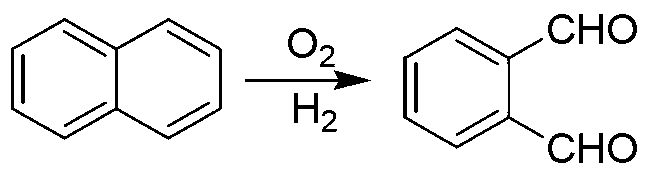
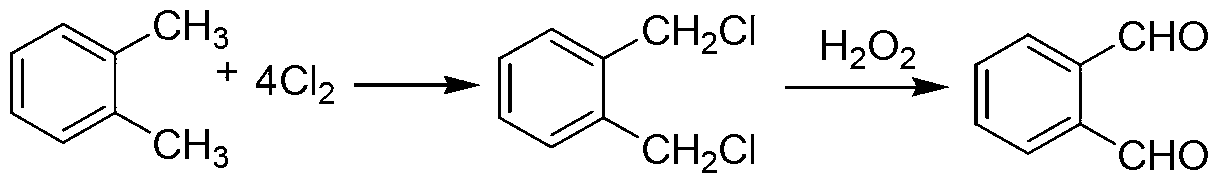
Green preparation technology of phthalic dicarboxaldehyde
A technology for the preparation of o-phthalaldehyde, which is applied in the field of green preparation technology of o-phthalaldehyde, can solve the problems of low yield of intermediate products, high product cost, and large environmental pollution, and achieve short reaction time and simple preparation process , the effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 0.10mol of 1,3-dihydroisobenzofuran, 0.20mol of hydrogen peroxide, 0.3g of phosphomolybdenum heteropolyacid and 10g of water into the three-necked flask in sequence, put in an electromagnetic stirrer, install a reflux condenser and After the temperature probe, set the reaction temperature to 30°C, ultrasonic power to 200W, and run for 30 minutes. After the reaction is completed, cool down to room temperature, extract with an equal volume of ethyl acetate for 3 times, combine the extracts, and remove the acetic acid in the extract by distillation. Ethyl ester, to obtain the crude product of phthalaldehyde, mix the crude product of phthalaldehyde with the recrystallization solvent petroleum ether according to the mass ratio of 1:3, stir under reflux for 15min, drop to 10°C, and obtain phthalaldehyde by suction filtration For formaldehyde crystals, the phthalaldehyde crystals were placed in a vacuum drying oven with a vacuum degree of 60 Pa and a temperature of 50°C for...
Embodiment 2
[0034] Add 0.10mol of 1,3-dihydroisobenzofuran, 0.20mol of hydrogen peroxide, 0.3g of phosphomolybdenum heteropolyacid and 10g of water into the three-necked flask in sequence, put in an electromagnetic stirrer, install a reflux condenser and After the temperature probe, set the reaction temperature to 35°C, the ultrasonic power to 250W, and run for 30 minutes. After the reaction is completed, cool down to room temperature, use an equal volume of ethyl acetate to extract 3 times, combine the extracts, and remove the acetic acid in the extracts by distillation. Ethyl ester, to obtain the crude product of phthalaldehyde, mix the crude product of phthalaldehyde with the recrystallization solvent petroleum ether according to the mass ratio of 1:3, stir under reflux for 15min, drop to 10°C, and obtain phthalaldehyde by suction filtration For formaldehyde crystals, the phthalaldehyde crystals were placed in a vacuum drying oven with a vacuum degree of 60 Pa and a temperature of 50 °C...
Embodiment 3
[0036] Add 0.10mol of 1,3-dihydroisobenzofuran, 0.26mol of hydrogen peroxide, 0.5g of phosphomolybdenum heteropolyacid, and 10g of water in sequence in the three-necked flask, put it into an electromagnetic stirrer, and install a reflux condenser and a temperature probe. , set the reaction temperature at 40°C, ultrasonic power at 300W, and run for 40min. After the reaction was completed, cool down to room temperature, extract three times with an equal volume of ethyl acetate, combine the extracts, remove ethyl acetate by distillation, and obtain phthalate The crude product of formaldehyde is recrystallized with petroleum ether for the crude product of o-phthalaldehyde, and is vacuum-dried to obtain the product of o-phthalaldehyde (the specific steps are the same as in Example 1), and the yield is 43%. The petroleum ether is reclaimed by distillation, and the petroleum ether recovered reusable.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com