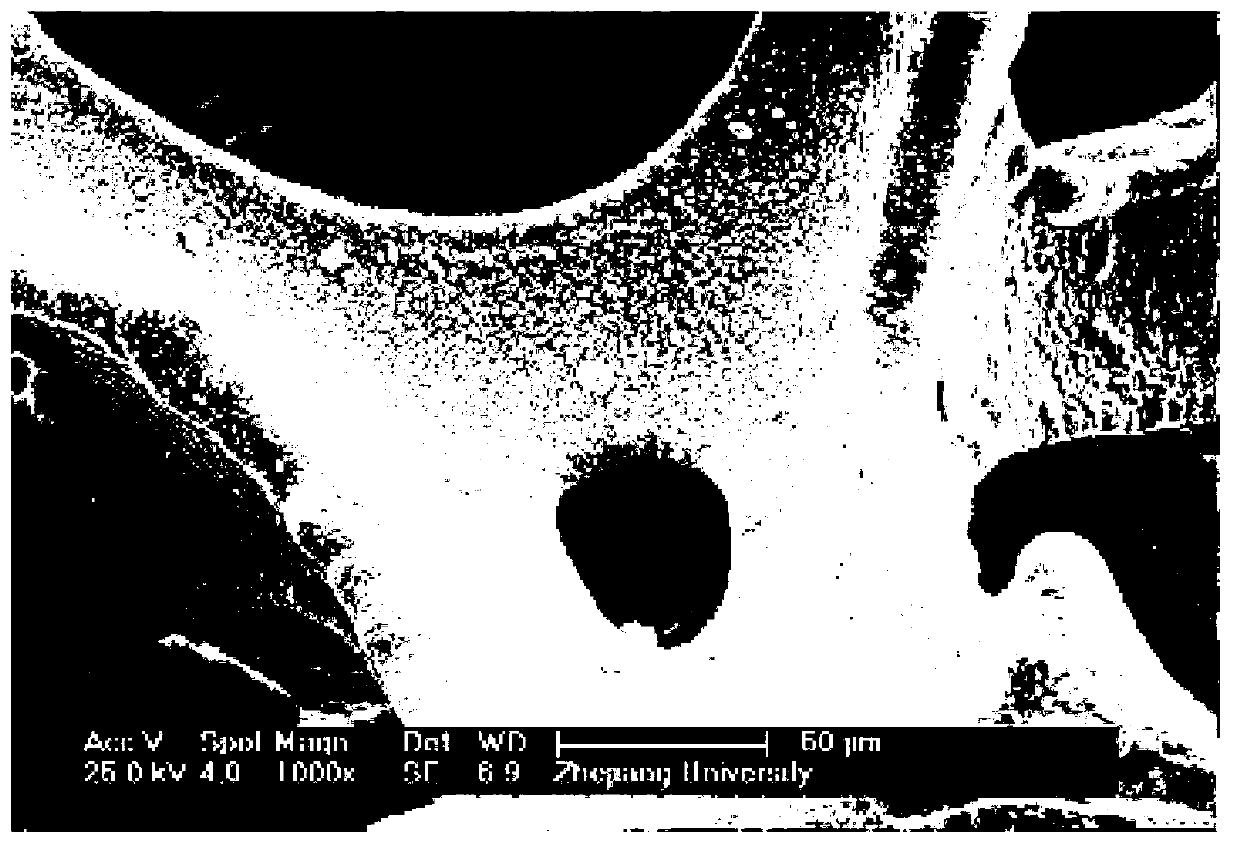
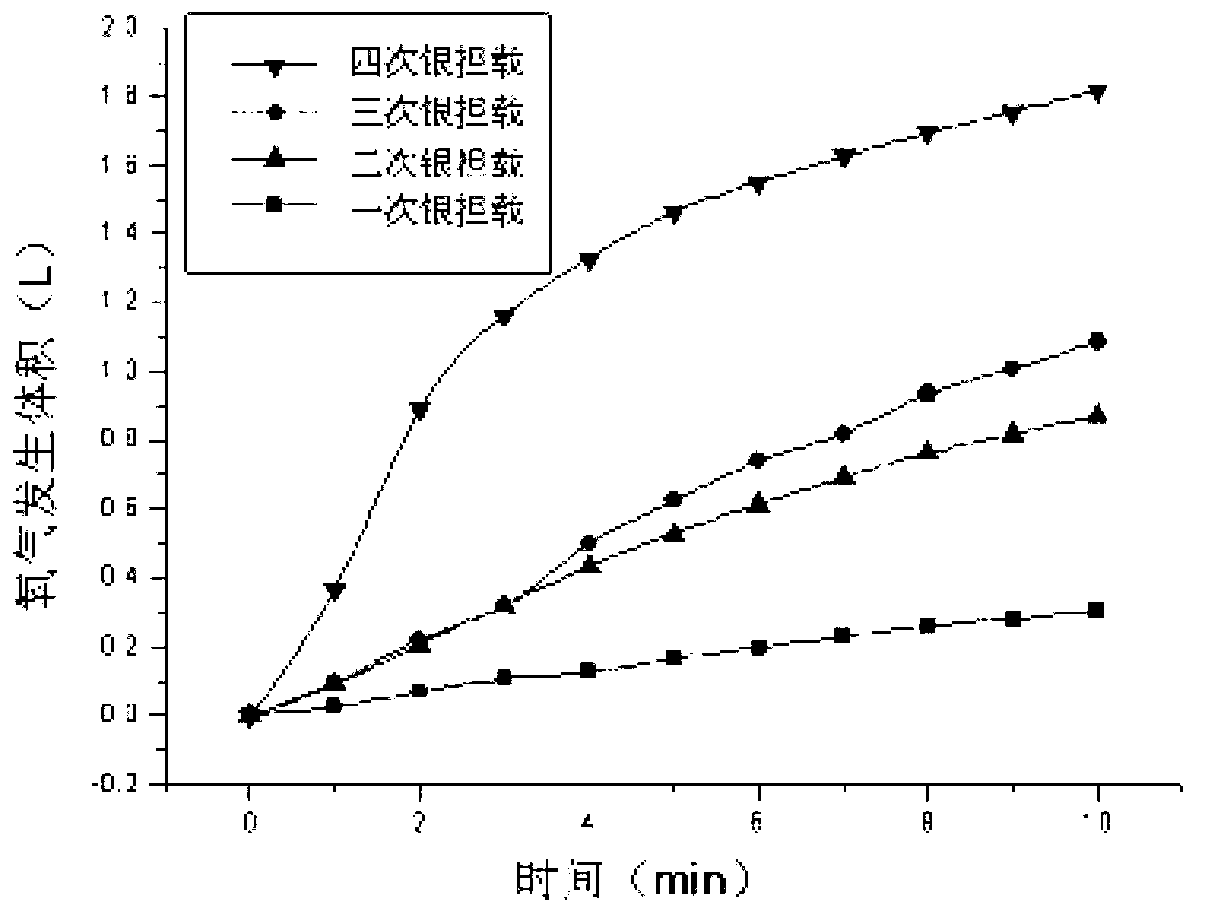
Preparation method of catalysts for oxygen production implemented through decomposition of hydrogen peroxide
A technology of catalyst and hydrogen peroxide, applied in metal/metal oxide/metal hydroxide catalyst, oxygen preparation, catalyst carrier, etc. Oxygen system reliability, catalyst and carrier detachment, etc., to achieve the effect of improving life, increasing shock wave ability, and enhancing mechanical strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: silver loading
[0024] Dissolve silver nitrate in water to prepare 20 milliliters of silver nitrate solution with a concentration of 0.5 wt% and introduce it into a test tube. Immerse a round rod-shaped nickel foam with a hole diameter of 0.5 mm, a diameter of 1 cm and a height of 5 cm in the silver nitrate solution of the test tube. The temperature of the test tube was raised to 40°C, the displacement reaction was carried out for 2 hours, and then cooled to room temperature.
Embodiment 2
[0025] Embodiment two: Nickel re-deposition
[0026] Dissolve silver nitrate in water to prepare 20 ml of a silver nitrate solution with a concentration of 3 wt%, and introduce it into a test tube, and immerse a nickel foam sheet with a pore size of 0.1 mm, a length of 10 cm, a width of 2 cm, and a thickness of 1 mm in the silver nitrate solution of the test tube , the temperature of the test tube was raised to 60°C, the displacement reaction was carried out for 1 hour, and then cooled to room temperature.
[0027] Sodium borohydride was dissolved in a sodium hydroxide solution with a concentration of 5 wt% to prepare an alkaline sodium borohydride solution with a concentration of 4 wt%, and 15 ml was introduced into the above-mentioned test tube. Sodium borohydride reduces the Ni ions in the solution to metallic nickel and deposits it on the nickel foam.
Embodiment 3
[0028] Embodiment three: calcining
[0029] Dissolve silver nitrate in water to prepare 20 ml of silver nitrate solution with a concentration of 1 wt%, introduce it into a test tube, immerse a spherical nickel foam with a pore size of 1 mm and a diameter of 1 cm in the silver nitrate solution of the test tube, and raise the temperature of the test tube to 80 °C , carried out the displacement reaction for 1.5 hours, and cooled to room temperature.
[0030] Sodium borohydride was dissolved in a sodium hydroxide solution with a concentration of 5 wt% to prepare an alkaline sodium borohydride solution with a concentration of 4 wt%, and 15 ml was introduced into the above-mentioned test tube. Sodium borohydride reduces the Ni ions in the solution to metallic nickel and deposits it on the nickel foam.
[0031] Take out the nickel foam, wash it, and dry it. The sample was placed in a muffle furnace and calcined at 400° C. for 4 hours under the protection of a nitrogen atmosphere to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com