Vitamin C freeze-dried powder for injection and preparation method thereof
A technology of freeze-dried powder injection and vitamins, which is applied in the field of medicine, can solve the problems of clinical drug safety, unsafe environment, large quantities, etc., and achieve the effects of favorable drug efficacy, energy saving, and fast forming
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
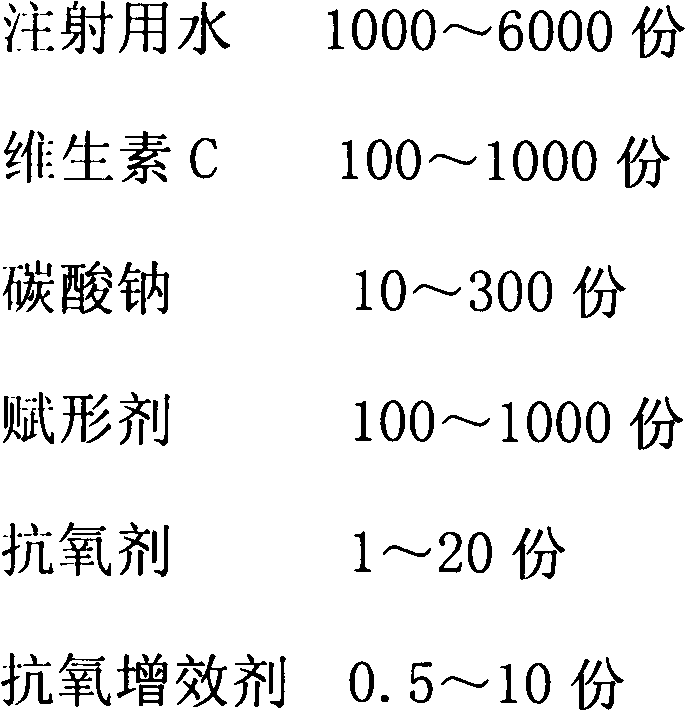
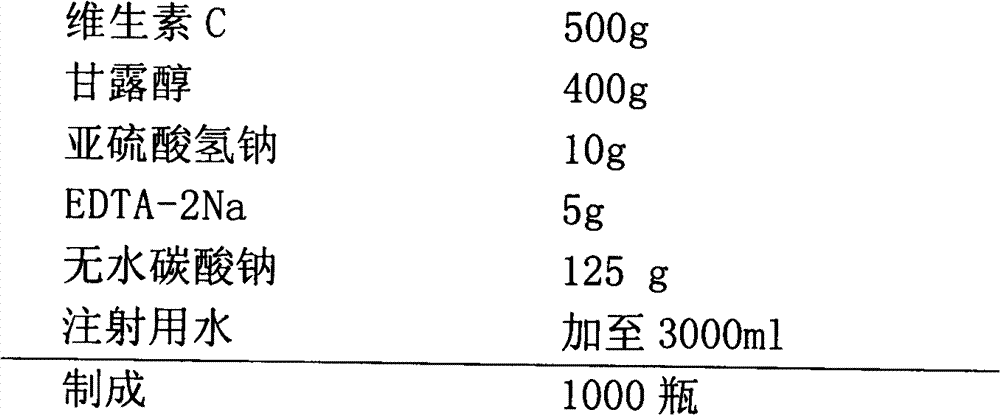
[0038]
[0039] Production Process:
[0040] 1).Preparation of liquid medicine: 60% of the prescription amount of water for injection is saturated with carbon dioxide, add antioxidant and antioxidant synergist and stir to dissolve, then add vitamin C to dissolve, add sodium carbonate to fully stir to dissolve, and wait until the bubbles are exhausted Stir for 30 minutes, add mannitol, stir to dissolve.
[0041] 2). Depyrogenation: add 0.15% medicinal activated carbon, stir and adsorb for 30 minutes, decarbonize by coarse filtration, add water for injection filled with carbon dioxide in advance to the full amount, and test the intermediate product.
[0042] 3). Sterilization and filtration: After passing the inspection, filter with a 0.22-micron microporous membrane, and carry out under a local laminar flow hood under the 10,000-level.
[0043] 4). Filling: Adjust the filling volume to 2ml, and divide into controlled antibiotic glass bottles. The liquid is filled with carbo...
Embodiment 2
[0052]
[0053] Production Process:
[0054]1).Preparation of liquid medicine: 60% of the prescription amount of water for injection is saturated with carbon dioxide, add antioxidant and antioxidant synergist and stir to dissolve, then add vitamin C to dissolve, add sodium carbonate to fully stir to dissolve, and wait until the bubbles are exhausted Stir for 30 minutes, add mannitol, stir to dissolve.
[0055] 2). Depyrogenation: add 0.15% medicinal activated carbon, stir and adsorb for 30 minutes, decarbonize by coarse filtration, add water for injection filled with carbon dioxide in advance to the full amount, and test the intermediate product.
[0056] 3). Sterilization and filtration: After passing the inspection, filter with a 0.22-micron microporous membrane, and carry out under a local laminar flow hood under the 10,000-level.
[0057] 4). Filling: adjust the filling volume to 3ml, and divide it into controlled antibiotic glass bottles. The liquid medicine is inflat...
Embodiment 3
[0066]
[0067] Production Process:
[0068] 1).Preparation of liquid medicine: 60% of the prescription amount of water for injection is saturated with carbon dioxide, add antioxidant and antioxidant synergist and stir to dissolve, then add vitamin C to dissolve, add sodium carbonate to fully stir to dissolve, and wait until the bubbles are exhausted Stir for 30 minutes, add mannitol, stir to dissolve.
[0069] 2). Depyrogenation: add 0.15% medicinal activated carbon, stir and adsorb for 30 minutes, decarbonize by coarse filtration, add water for injection filled with carbon dioxide in advance to the full amount, and test the intermediate product.
[0070] 3). Sterilization and filtration: After passing the inspection, filter with a 0.22-micron microporous membrane, and carry out under a local laminar flow hood under the 10,000-level.
[0071] 4). Filling: Adjust the filling volume to 6ml, and divide into controlled antibiotic glass bottles. The liquid is filled with carbo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com