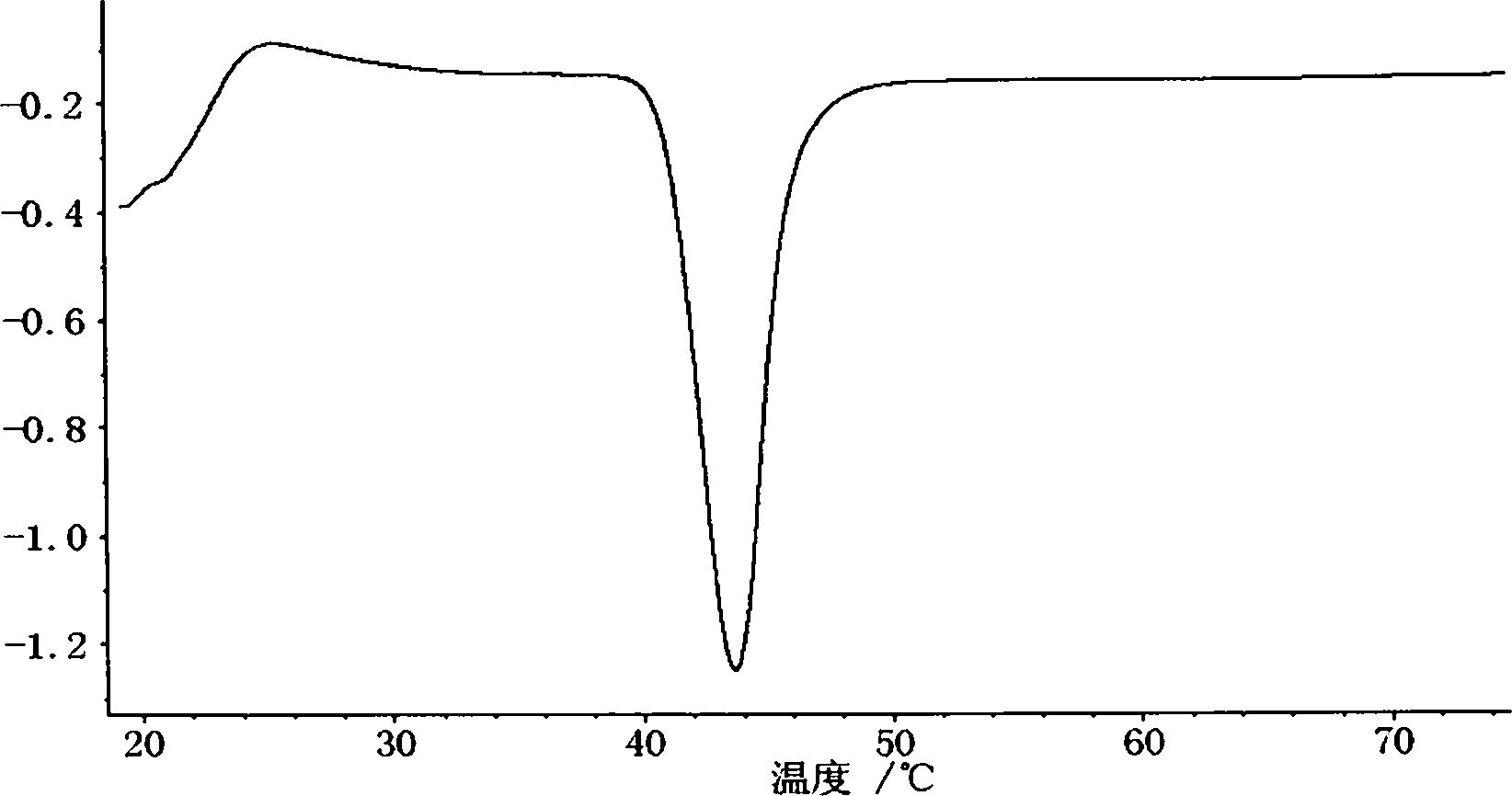
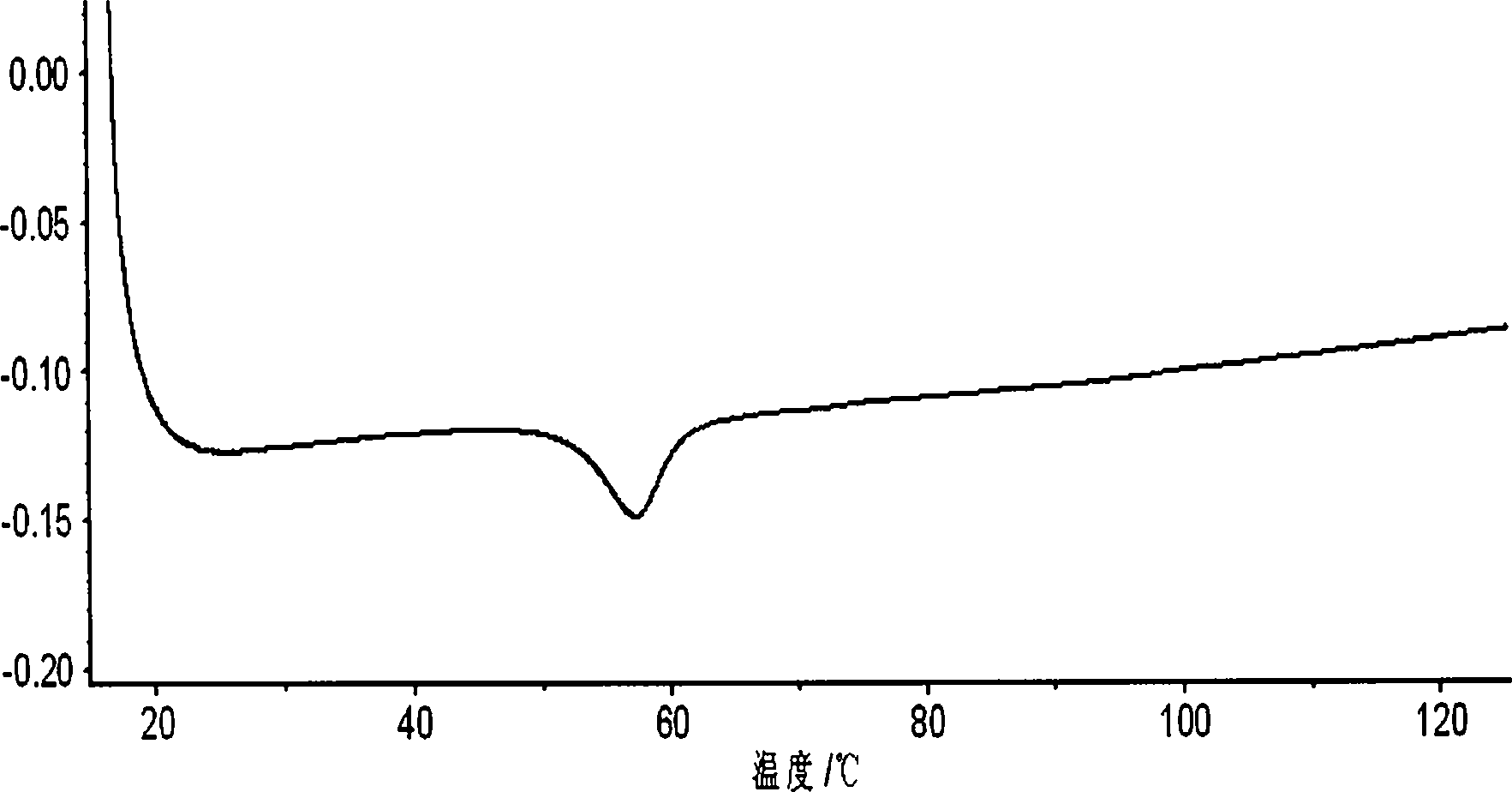
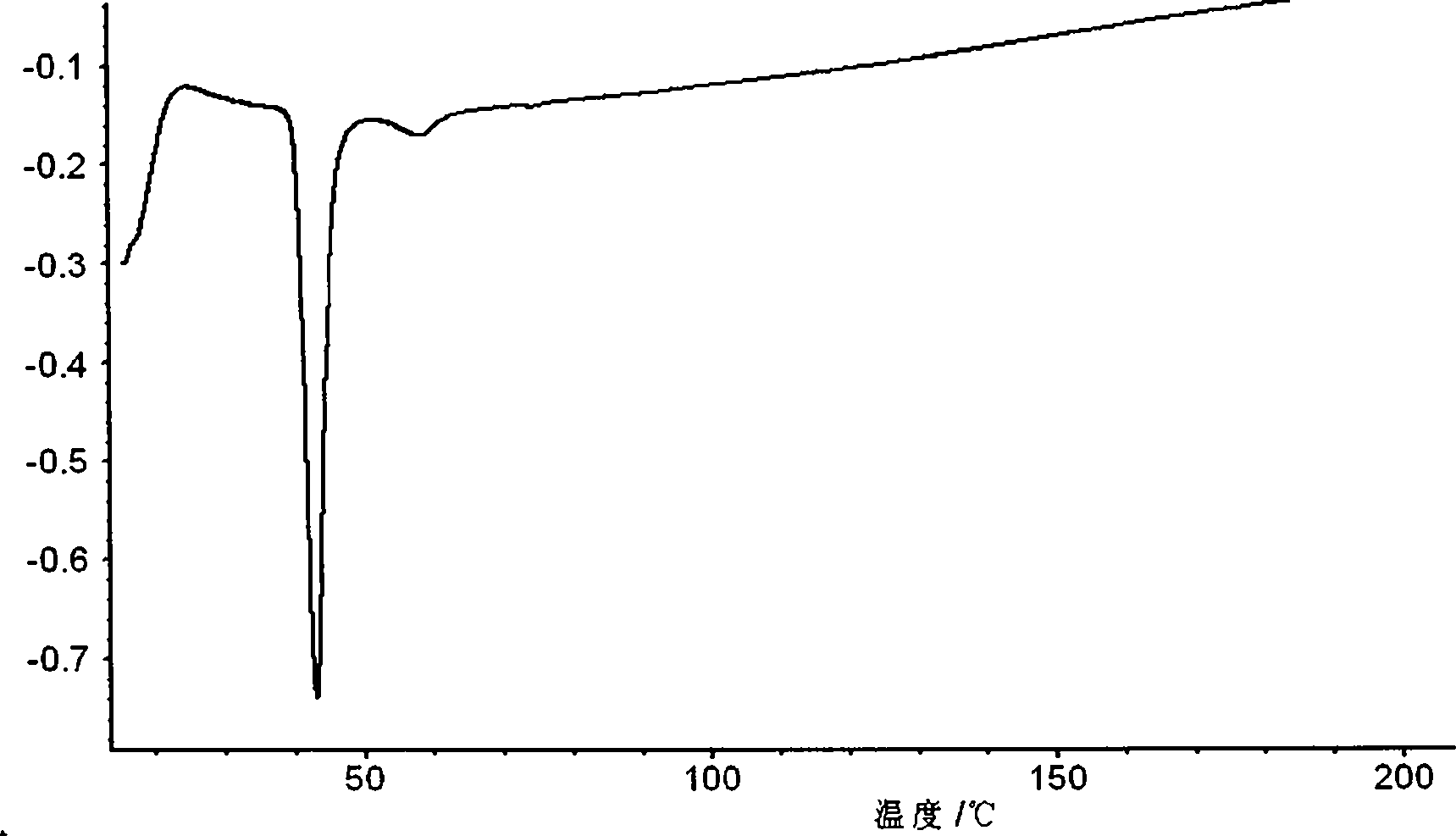
Orlistat oral preparation and preparation method thereof
A technology of orlistat and tablets, which is applied in the field of pharmaceutical preparations and can solve the problems of tablet dissolution, stickiness, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1. Solid Dispersion Preparation
[0034] Orlistat 120g
[0035] Acrylic resin 60g
[0036] Absolute ethanol 600g
[0037] Dissolve the prescribed amount of orlistat and acrylic resin in absolute ethanol, and on the supercritical fluid equipment, pass the supercritical fluid CO2 to quickly depressurize through a nozzle with an aperture of 10 microns, and the decompression time is 10 -5 Second, a solid dispersion of orlistat acrylic resin was prepared by supercritical fluid rapid expansion method, and the D90 of the dispersion was less than 10 μm.
[0038] 2. Preparation of Orlistat Tablets
[0039]
[0040] Mix the solid dispersion with microcrystalline cellulose and crospovidone evenly, add an appropriate amount of pure water, granulate, dry, and pass the dry granules through a 20-mesh sieve, then mix evenly with the prescribed amount of magnesium stearate, and tablet it. have to.
Embodiment 2
[0042] 1. Solid Dispersion Preparation
[0043] Orlistat 120g
[0044] Acrylic resin 360g
[0045] Absolute ethanol 2000g
[0046]Dissolve the prescribed amount of orlistat and acrylic resin in absolute ethanol, and on the supercritical fluid equipment, pass the supercritical fluid CO2 to quickly depressurize through a nozzle with an aperture of 10 microns, and the decompression time is 10 -5 Second, a solid dispersion of orlistat acrylic resin was prepared by supercritical fluid rapid expansion method, and the D90 of the dispersion was less than 10 μm.
[0047] 2. Preparation of Orlistat Tablets
[0048]
[0049] Mix the solid dispersion with microcrystalline cellulose and crospovidone evenly, add an appropriate amount of pure water, granulate, dry, and pass the dry granules through a 20-mesh sieve, then mix evenly with the prescribed amount of magnesium stearate, and tablet it. have to.
Embodiment 3
[0051] 1. Solid Dispersion Preparation
[0052] Orlistat 120g
[0053] Acrylic resin 180g
[0054] Absolute ethanol 1000g
[0055] Dissolve the prescribed amount of orlistat and acrylic resin in absolute ethanol, and on the supercritical fluid equipment, pass the supercritical fluid CO2 to quickly depressurize through a nozzle with an aperture of 10 microns, and the decompression time is 10 -5 Second, a solid dispersion of orlistat acrylic resin was prepared by supercritical fluid rapid expansion method, and the D90 of the dispersion was less than 10 μm.
[0056] 2. Preparation of Orlistat Tablets
[0057]
[0058] Mix the solid dispersion with microcrystalline cellulose and crospovidone evenly, add an appropriate amount of pure water, granulate, dry, and pass the dry granules through a 20-mesh sieve, then mix evenly with the prescribed amount of magnesium stearate, and tablet it. have to.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com