Separation determination method of rivaroxaban optical isomer
A technology for optical isomers and rivaroxaban, which is applied in the field of separation and determination of optical isomers of rivaroxaban, can solve the problems of losing chiral recognition ability and destroying spatial structure, achieving good separation and accurate results , the effect of high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A method for separating and measuring optical isomers of rivaroxaban, specifically comprising the steps of:
[0033] (1) Chromatographic separation of optical isomers of rivaroxaban
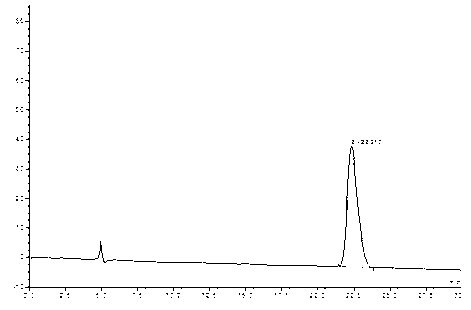
[0034] Weigh 10 mg of the rivaroxaban sample (S configuration) to be tested, use a 10 ml volumetric flask to set the volume to the mark, shake well, and use it as the test solution 1, the injection flow rate is 1.0 ml / min, and the detection wavelength is 250 nm; Select an IC chiral chromatographic column, the column temperature is 30°C, the injection volume is 5 μl, the mobile phase is acetonitrile=100%, and the flow rate of the mobile phase is set to 0.7ml / min to complete the separation of the optical isomers of rivaroxaban to be tested;
[0035] (2) Determination of the content of optical isomers of rivaroxaban
[0036] Weigh about 10 mg of rivaroxaban racemate standard sample, dissolve it with acetonitrile, use a 10 ml volumetric flask to adjust the volume to the mark, shake well, and ...
Embodiment 2
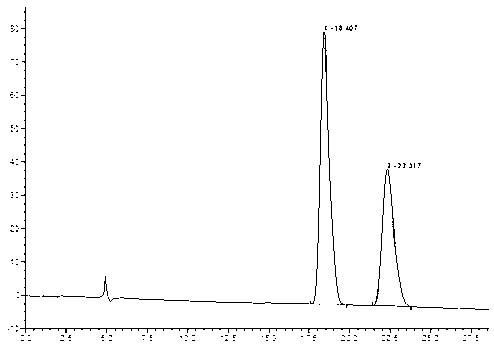
[0043] Take the test solution 2 obtained in Example 1 respectively, and according to the chromatographic conditions, the detection wavelength: 250nm, column temperature: 30°C, flow rate: 0.7ml / min; mobile phase: acetonitrile-water=40-60 (V / V) Carry out chromatographic analysis, record the chromatogram, see the results figure 2 ;
[0044] figure 2 The chromatographic peak at 31.562min is the chromatographic peak of rivaroxaban in S configuration, and the chromatographic peak at 27.688min is the chromatographic peak of rivaroxaban in R configuration in the optical isomer of rivaroxaban.
[0045] The chromatogram of the obtained rivaroxaban optical isomer sample (S configuration) was compared with the separation chromatogram of the rivaroxaban racemate standard sample obtained in step (2) of Example 1 by using the area normalization method , so that the content of rivaroxaban in S configuration in the optical isomers of rivaroxaban to be tested is 38%, and the content of rivaro...
Embodiment 3
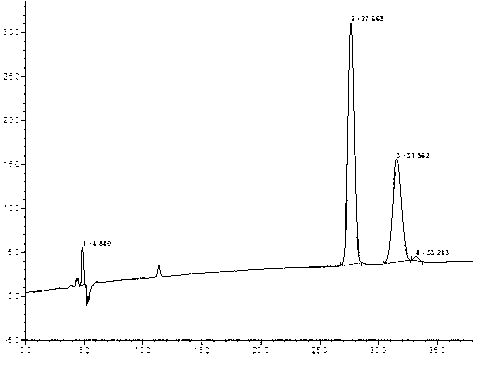
[0048] Take the test solution 2 obtained in Example 1 respectively, and according to the chromatographic conditions, the detection wavelength: 250nm, column temperature: 30°C, flow rate: 0.7ml / min; mobile phase: acetonitrile-water=90-10 (V / V) Carry out chromatographic analysis, record the chromatogram, see the results image 3 ;
[0049] image 3 The chromatographic peak at 9.547min is the chromatographic peak of rivaroxaban in S configuration, and the chromatographic peak at 8.877min is the chromatographic peak of rivaroxaban in R configuration, which is the optical isomer of rivaroxaban.
[0050] The chromatogram of the obtained rivaroxaban optical isomer sample (S configuration) was compared with the separation chromatogram of the rivaroxaban racemate standard sample obtained in step (2) of Example 1 by using the area normalization method , so that the content of rivaroxaban in S configuration in the optical isomers of rivaroxaban to be tested is 38%, and the content of r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com