Dengue virus degeneration vaccine and application thereof
A dengue virus and vaccine technology, applied in antiviral immunoglobulins, applications, viral peptides, etc., can solve the problem that the type 2 dengue virus strain cannot play a protective role, the protection effect of specific epidemic strains is poor, and the protective power is relatively different. Large and other problems, to achieve the effects of low mammalian cell expression system, cost control, and improved safety of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Design of Dengue Virus Degeneracy Sequence Vaccine Molecules
[0045] 1. Sequence Alignment
[0046] DV is widely popular in tropical and subtropical regions, especially in Southeast Asia, Africa, Central and South America and the Western Pacific. The present invention selects DV strains that have been popular in the world, and the DV strains include representative strains of four serotypes of DV-1, DV-2, DV-3 and DV-4. DV-1 selects the epidemic strains in the Southwest Indian Ocean and Africa (GenBank accession number DQ285558), the Indonesian epidemic strains (GenBank accession number AB189121), the American strains (GenBank accession number GQ868530) and the Chinese epidemic strains (GenBank accession number AY376738); DV-1 2 Select the TR1751 strain popular in the United States and Japan (Trent, et al.1983), the popular strain in West Africa (GenBank accession No. EF105386 and EF105378), the American strain (GenBank accession No. HM582117); DV-3 selec...
Embodiment 2
[0063] Example 2 Construction of Dengue Virus Degenerate Vaccine Recombinant Expression Plasmid
[0064] 1. BamHI (TaKaRa Company) and XhoI (TaKaRa Company) double enzyme digestion of chemically synthesized degenerate sequence DVIII
[0065]
[0066] After mixing, put it in a water bath at 37°C for 4 hours, run 1.2% agarose (Shanghai Sangong) gel electrophoresis, and recover the double-digested DVIII fragment according to the instructions of the Promega gel recovery kit;
[0067] 2. The prokaryotic expression vector pET22b was double-digested by BamHI (TaKaRa Company) and XhoI (TaKaRa Company), and the double-enzyme digestion reaction system was the same as above, and the plasmid was recovered;
[0068]
[0069] After mixing, put it in a water bath at 16°C for 16 hours, and inactivate the ligase at 65°C for 10 minutes;
[0070] 4. Transformation experiments
[0071] For the preparation of Escherichia coli DH5α competent cells, refer to the Molecular Cloning Experime...
Embodiment 3
[0072] Example 3 Screening and Characterization of Recombinant Bacterial Clones
[0073] 1. Transform the recombinant expression plasmid pET22b-DVIII described in Example 2 into a competent BL21 Escherichia coli according to the Molecular Cloning Experiment Guide (Third Edition, Science Press, 2002). For details, refer to the description in step 4 in Example 2 method is carried out.
[0074] 2. Pick 10 AMP-resistant clones and culture them with 1ml LB medium. When the OD600 value is about 0.3-0.5, add 0.5mM IPTG (Beijing Saibaisheng) inducer to induce for 1 hour, and centrifuge at 14000 rpm for 5 Minutes, collect the bacteria.
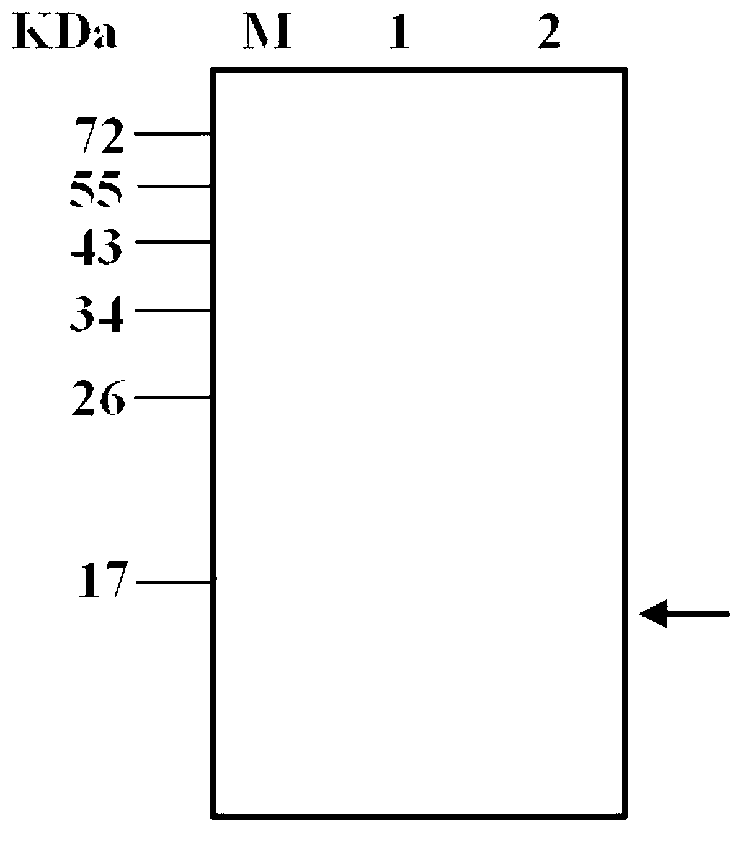
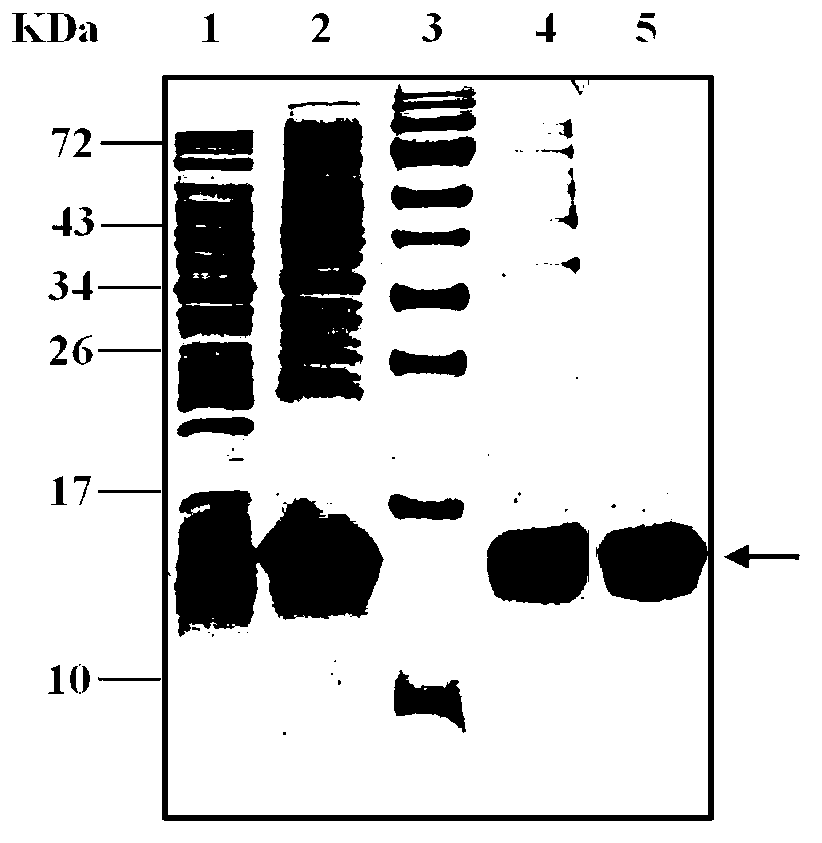
[0075] 3. Carry out SDS-PAGE electrophoresis (Bio-Rad Company, USA) according to the method of the molecular cloning experiment guide, and the gel after electrophoresis is stained with Coomassie Blue (Fluka Company, Switzerland). The clones with clear expression bands were expression-positive clones, named as pET22b-DVIII / BL21 engineering bacteria...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com