Catalyst for norbornene monomer polymerization and method for producing norbornene polymer
A norbornene and manufacturing method technology, applied in the field of new transition metal coordination compounds, can solve the problems of high activity and small activity reduction, unknown, low polymerization activity, etc., and achieve the effect of excellent transparency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0157] Hereinafter, although an Example and a comparative example are given and this invention is demonstrated in more detail, this invention is not limited by these description at all.
[0158] In each embodiment and comparative example, catalytic activity is calculated by following formula:
[0159] [number 1]
[0160] Catalytic activity = (amount of polymer obtained [g]) / (moles of palladium [mmol])
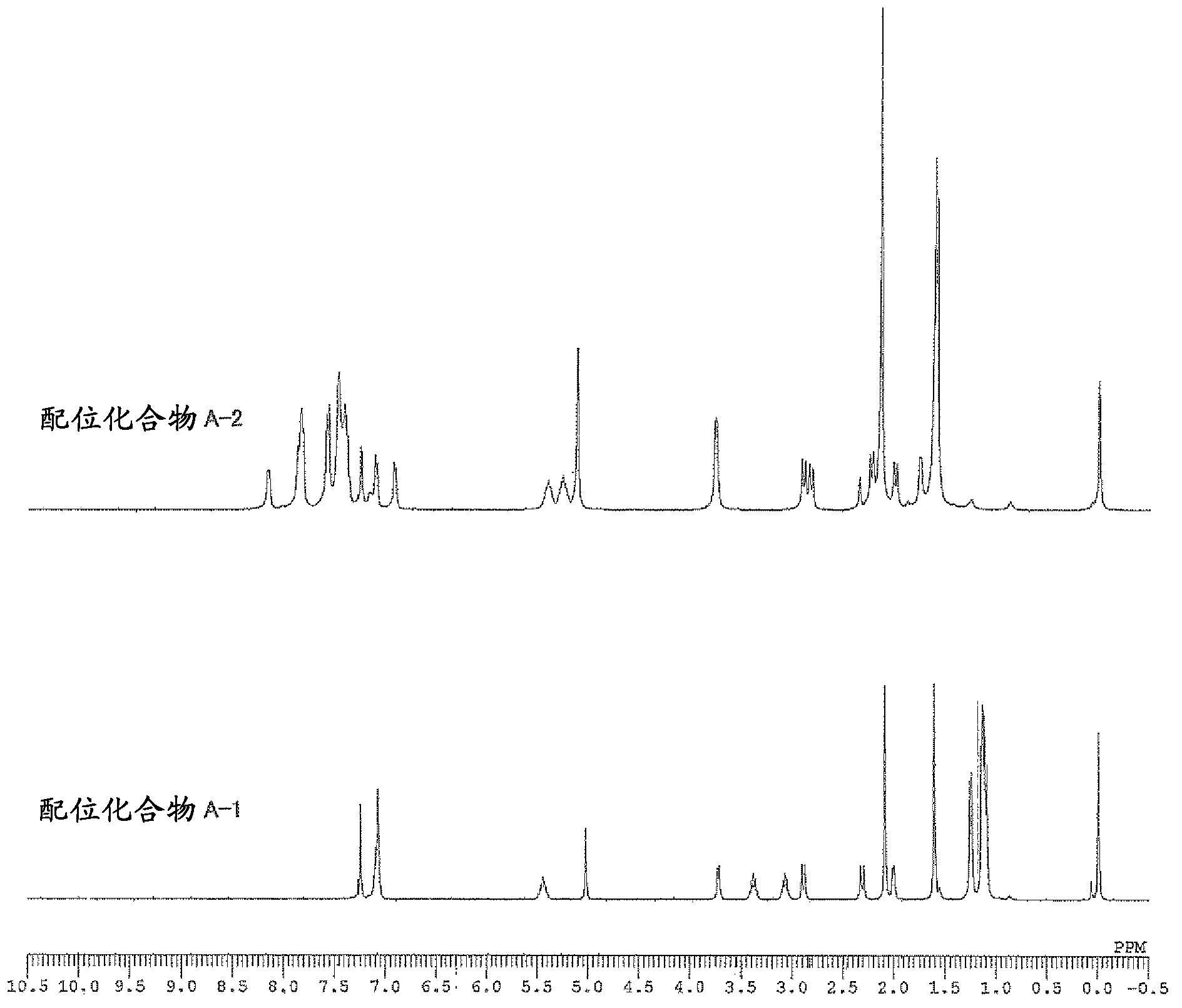
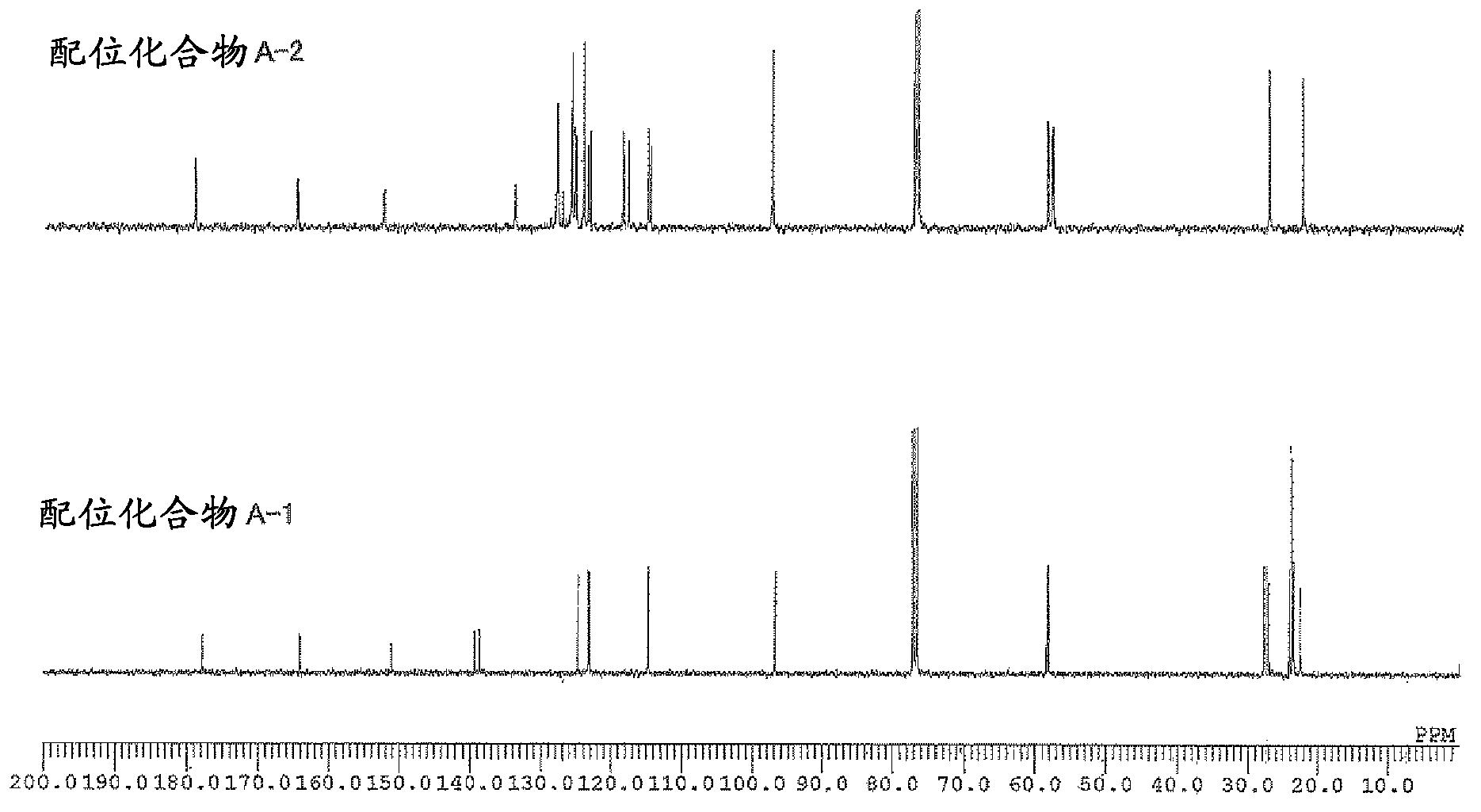
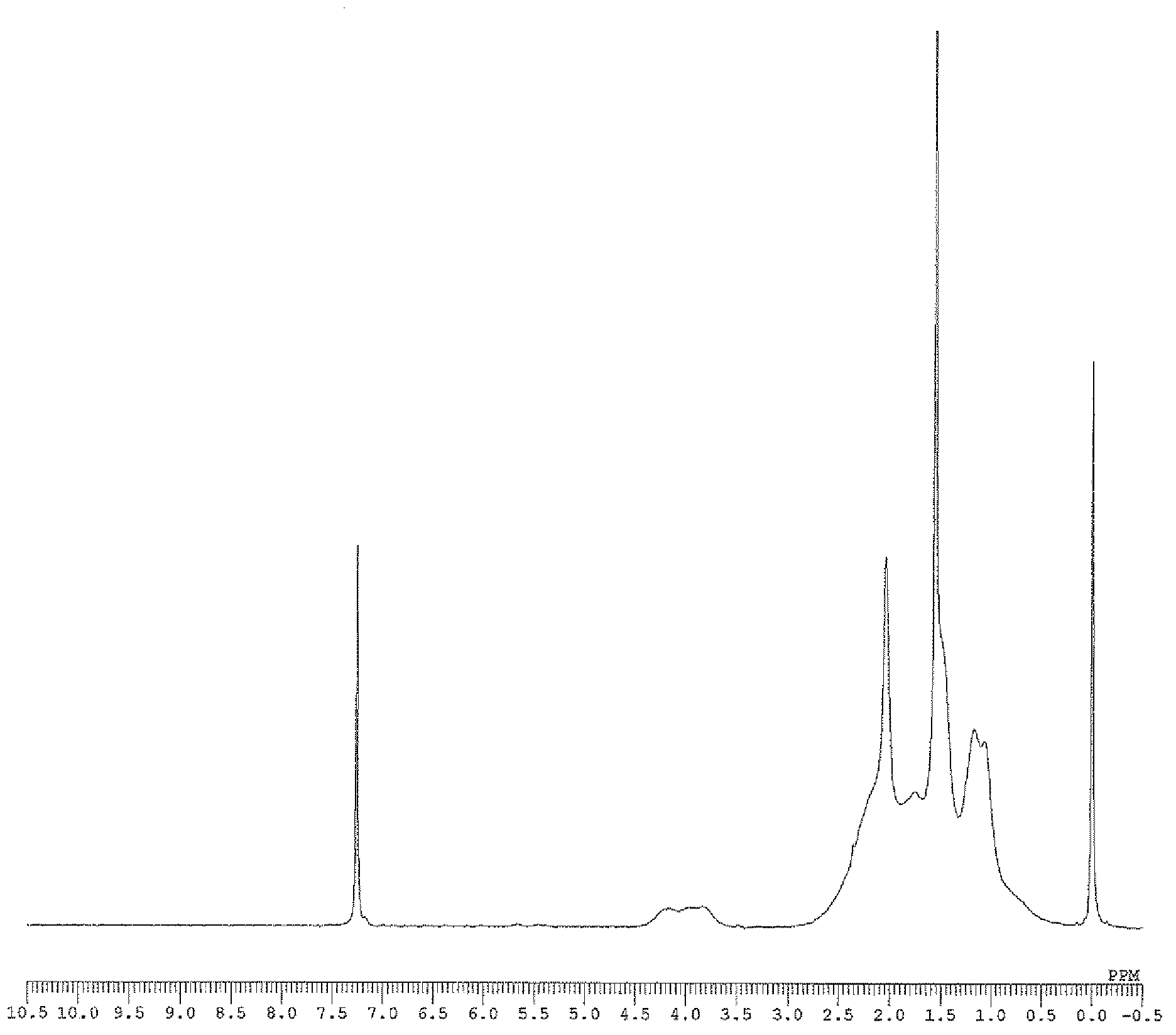
[0161] The weight average molecular weight (Mw), number average molecular weight (Mn), and molecular weight distribution (Mw / Mn) of the obtained polymer were determined by gel permeation chromatography (GPC) using polystyrene as a standard substance. In addition, the composition ratio of norbornene to 5-acetoxymethyl-2-norbornene in the copolymer was determined by 1 The peak obtained by H-NMR [δ: 3.5-4.5ppm, 5-acetoxymethyl-2-norbornene (abbreviated as "ANB") "-COOCH 2 -" unit] and [δ: 0.5-3.0ppm, norbornene (abbreviated as "NB") and "CH of 5-acetoxymethyl-2-norbornene 3 CO...
Synthetic example 1
[0187] Synthesis Example 1: Synthesis of 2-Acetoxymethyl-5-Norbornene
[0188] Dicyclopentadiene (manufactured by Tokyo Chemical Industry Co., Ltd., 759.80 g, 5.747 mol), allyl acetate (manufactured by Tokyo Chemical Industry Co., Ltd., 1457.86 g, 14.561 mol) and hydroquinone (Wako Pure Pharmaceutical Co., Ltd., 2.25 g, 0.0204 mol). After replacing the inside of the system with nitrogen, the autoclave was heated up to 190° C. while stirring at 500 rpm, and reacted for 5 hours. After the reaction, the autoclave was cooled to room temperature, the contents were transferred to a distillation apparatus, and distillation was performed under reduced pressure to obtain 1306.70 g of a colorless transparent liquid as a fraction at 0.07 kPa and 48°C.
[0189] Measure the resulting liquid 1 H-NMR confirmed the target 2-acetoxymethyl-5-norbornene. In addition, the molar ratio of the exo isomer to the endo isomer of the obtained 2-acetoxymethyl-5-norbornene was exo / endo=18 / 82.
Embodiment 1
[0190] Example 1: (π-allyl){4-(2,6-diisopropylphenylimino)-2-pentene-2-alcohol radical-κ 2 Synthesis of N, O} Palladium [Coordination Compound A-1]
[0191]
[0192] [Coordination compound A-1]
[0193] A two-necked flask equipped with a three-way valve was replaced with nitrogen, and 4-(2,6-diisopropylphenylimino)pentane-2-one (471.6mg, 1.818mmol) was added thereto, and dehydrated tetrahydrofuran ( Wako Pure Chemical Industries, Ltd., 10 ml) was dissolved. After immersing it in a dry ice-ethanol bath and cooling it to -78°C, add a 1.6mol / l hexane solution of n-butyllithium (manufactured by Wako Pure Chemical Industries, Ltd., 1.14ml, 1.82mmol) dropwise. After stirring at -78° C. for 1 hour, the temperature was gradually returned to room temperature.
[0194] A separately prepared two-necked flask equipped with a three-way valve was replaced with nitrogen, and allyl palladium chloride dimer (manufactured by Wako Pure Chemical Industries, Ltd., 329.1 mg, 0.900 mmol) was a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com