Cycloolefin copolymer and preparation method thereof
A technology of cyclic olefin copolymers and polymerization reaction products, which is applied in the field of copolymers, can solve the problems of poor heat resistance, poor mechanical properties, and poor elongation at break of cyclic olefin copolymers, and achieve good transparency, Good heat resistance and mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] The present invention provides a kind of preparation method of cycloolefin copolymer described in above-mentioned technical scheme, comprises the following steps:
[0047] 1), under the action of a catalyst, a compound having a structure shown in formula II and a compound having a structure shown in formula III are polymerized in a solvent to obtain a polymerized reaction product;
[0048] 2), hydrogenating the polymerization reaction product and a hydrogen source to obtain a cycloolefin copolymer;
[0049]
[0050] In the present invention, preferably, the compound having the structure shown in formula II, the compound having the structure shown in formula III and a solvent are mixed, and a catalyst is added to the obtained mixture for polymerization reaction to obtain a polymerization reaction product. In the present invention, the compound having the structure represented by formula II, the compound having the structure represented by formula III and the solvent a...
Embodiment 1
[0113] Add 800 mL of norbornadiene, 230 grams of anthracene and 1 gram of 2,6-di-tert-butyl-p-cresol in sequence to a 2-liter stainless steel autoclave, and repeatedly vacuumize the autoclave 3 times Nitrogen filling operation: the autoclave was heated to 180° C., and the contents in the autoclave were reacted for 30 hours under stirring conditions.
[0114] After the reaction, the obtained reaction product was cooled to 25° C., left to stand for 12 hours and then filtered. The obtained filtered product was washed twice with n-hexane to obtain 260 g of the product. The yield of the product prepared by the method provided in Example 1 of the present invention was 75%.
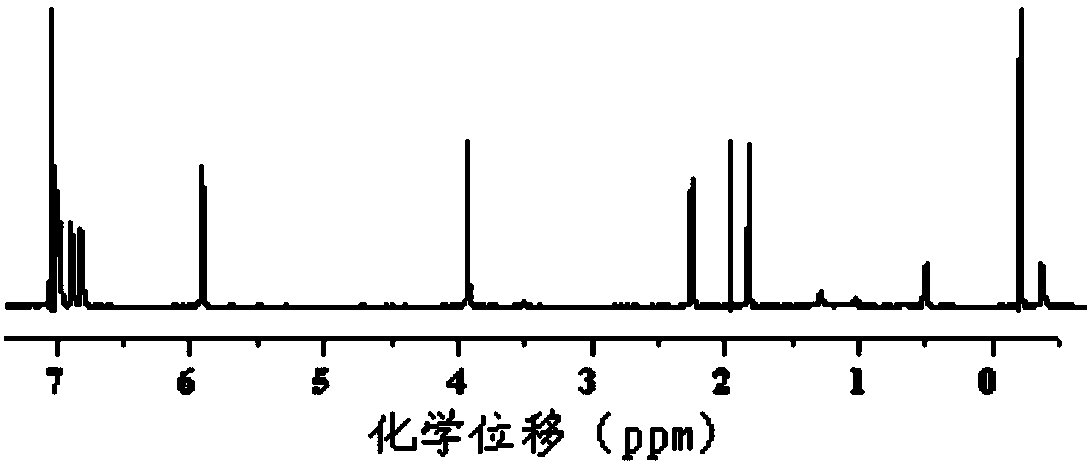
[0115] The above-mentioned obtained product is carried out to proton nuclear magnetic resonance spectrum detection, and detection result is as follows: figure 1 as shown, figure 1 For the proton nuclear magnetic resonance spectrum figure of the product that the embodiment of the present invention 1 obtains, by...
Embodiment 2
[0117] 300 mL of n-octene, 108 mL of dicyclopentadiene and 1 gram of 2,6-di-tert-butyl-p-cresol were successively added to a 2-liter stainless steel autoclave, and the vacuuming of the autoclave was repeated 3 times The operation of post-filling with nitrogen: the autoclave was heated to 240°C, and the contents in the autoclave were reacted for 8 hours under the condition of stirring.
[0118] After the reaction, the obtained reaction product was cooled to 25°C, and after standing for 12 hours, it was distilled at 120°C under normal pressure to collect unreacted n-octene, and then the resulting atmospheric distillation product was decompressed at 80°C Distill and collect fractions at 68°C to 80°C to obtain 108 grams of product. The yield of the product prepared by the method provided in Example 2 of the present invention is 38.9%.
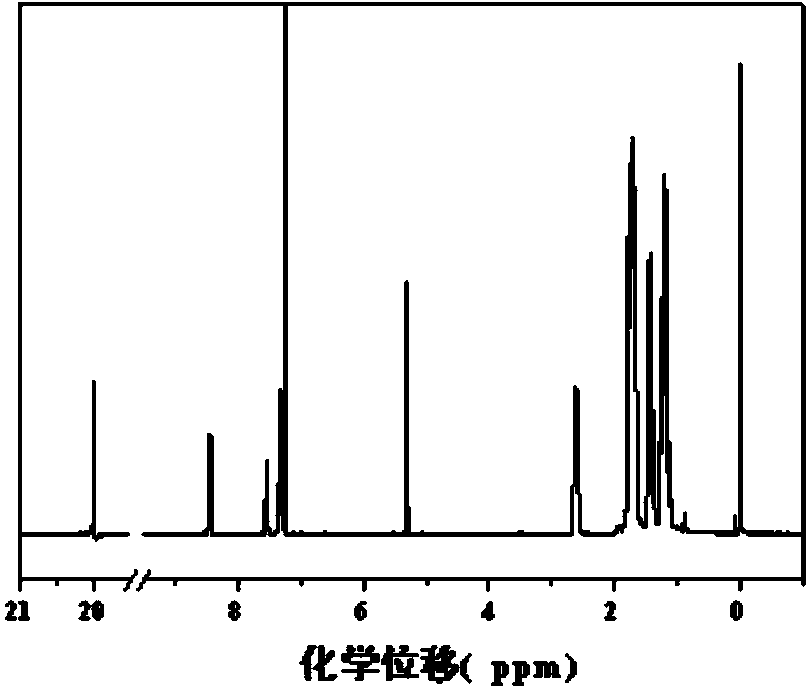
[0119] The above-mentioned obtained product is carried out to proton nuclear magnetic resonance spectrum detection, and detection result is as fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
| Tensile modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com