Erbium and terbium co-doped fluoride halide phosphate laser glass as well as preparation method and application thereof
A fluorohalophosphate and laser glass technology, applied in the field of fluorohalophosphate glass and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]According to the formula in Table 1, calculate the weight of the corresponding components, weigh the raw materials and mix them uniformly; put the mixture into a platinum crucible and melt it in a silicon carbide rod electric furnace at 900°C for 20 minutes to obtain molten glass. During the glass melting process, high-purity oxygen is always passed through for atmosphere protection to remove moisture in the molten glass. After the dehydrated glass liquid is homogenized and clarified, it is quickly poured into a mold that has been preheated to 360°C, and then quickly placed in a muffle furnace that has been heated to 410°C and kept for 2 hours; The muffle furnace is lowered to 100° C., then the muffle furnace is closed, and the temperature is lowered to room temperature to obtain the annealed erbium and terbium co-doped fluorohalophosphate glass.
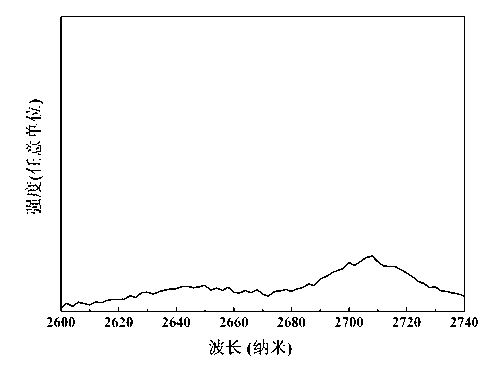
[0046] The annealed erbium and terbium co-doped fluorohalophosphate glass was processed into a 10×20×1 mm glass plate and po...
Embodiment 2
[0049] According to the formula in Table 1, calculate the weight of the corresponding components, weigh the raw materials and mix them evenly; put the mixture into a platinum crucible and melt it in a silicon carbide rod electric furnace at 950°C for 15 minutes to obtain molten glass. During the glass melting process, high-purity oxygen is always passed through for atmosphere protection to remove moisture in the molten glass. After being homogenized and clarified, the water-removed glass liquid is quickly poured into a mold that has been preheated to 370°C, and then quickly placed in a muffle furnace that has been heated to 420°C. The rate is lowered to 100° C., then the muffle furnace is closed, and the temperature is lowered to room temperature to obtain the annealed erbium and terbium co-doped fluorohalophosphate glass.
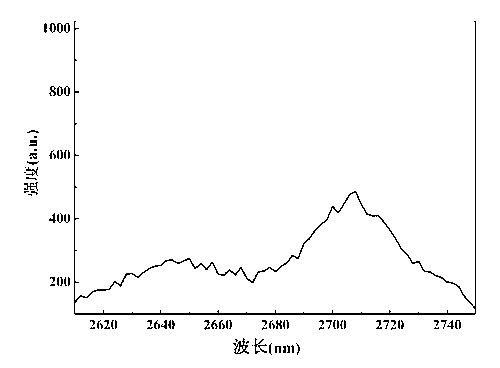
[0050] Take the erbium and terbium co-doped fluorohalophosphate glass after the above annealing, grind it into a fine powder with an agate mortar, and con...
Embodiment 3
[0056] According to the formula in Table 1, calculate the weight of the corresponding components, weigh the raw materials and mix them uniformly; put the mixture into a platinum crucible and melt it in a silicon carbide rod electric furnace at 1000°C for 18 minutes to obtain molten glass. During the glass melting process, high-purity oxygen is always passed through for atmosphere protection to remove moisture in the molten glass. After the dehydrated glass liquid is homogenized and clarified, it is quickly poured into a mold that has been preheated to 370°C, and then quickly placed in a muffle furnace that has been heated to 425°C, and kept for 3 hours, and then heated at a temperature of 9°C / hour. The rate was lowered to 110° C., then the muffle furnace was closed, and the temperature was lowered to room temperature to obtain the annealed erbium and terbium co-doped fluorohalophosphate glass.
[0057] The annealed erbium and terbium co-doped fluorohalophosphate glass was proc...
PUM
| Property | Measurement | Unit |
|---|---|---|
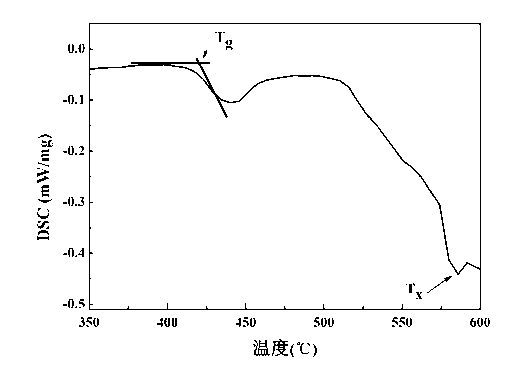
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com