Pharmaceutical composition, and preparation method and application thereof
A composition and drug technology, applied in the directions of drug combination, pharmaceutical formula, plant raw material, etc., can solve the problems of uncontrollable normal blood glucose level, weight gain, etc., and achieve the effects of novel formula, convenient taking and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Preparation of raw materials:
[0041] Fenugreek extract: extract fenugreek seeds at 60°C with ethanol with a mass concentration of 50% for 2-3 times, 1-3 hours each time; filter, discard the filter residue, and combine the filtrate; concentrate the filtrate to a relative density of 1.00 After -1.15, use 90% ethanol alcohol precipitation with 3 times the mass of the filter residue, and take its supernatant; the supernatant is adsorbed with an ion exchange resin for amino acids, then rinsed with deionized water, and then analyzed with 1N ammonia water. , to obtain the analysis solution; the analysis solution is concentrated and then dried to obtain the fenugreek extract;
[0042] Extract of plantago seed: reflux extract plantago seed with 75% ethanol aqueous solution with a mass concentration 4 times the mass of plantago seed for 3-5 times, each time for 1-2h; collect extract, filter, concentrate to remove solvent , and diluted with water to obtain a suspension solu...
Embodiment 2
[0047] (1) Preparation of raw materials:
[0048] Fenugreek extract: extract fenugreek seeds at 70°C with ethanol with a mass concentration of 60% for 2-3 times, 1-3 hours each time; filter, discard the filter residue, and combine the filtrate; concentrate the filtrate to a relative density of 1.15 Finally, use 85% ethanol alcohol precipitation with respect to 4 times the quality of the filter residue, and get its supernatant; the supernatant is adsorbed amino acids with ion exchange resin, then rinsed with deionized water, and then resolves the cationic resin column with 1.5N ammonia water, Obtain the analysis solution; the analysis solution is concentrated and then dried to obtain the fenugreek extract;
[0049] Plantago seed extract: use 80% ethanol aqueous solution with a mass concentration of 6 times the mass of Plantago seed to reflux extract the plantago seed for 5 times, each time for 1-2h; collect the extract, filter, concentrate to remove the solvent, add water Dilu...
Embodiment 3
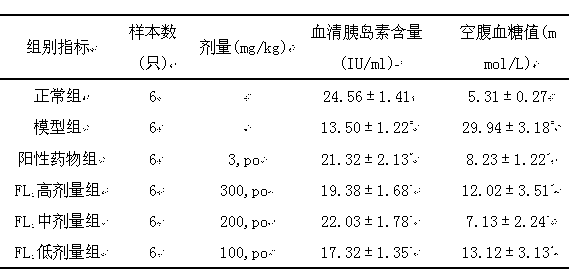
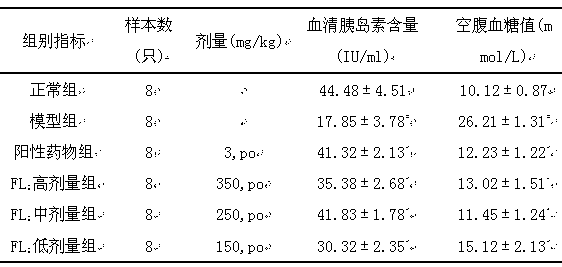
[0054] Healthy male mice (22±2) g were selected and randomly divided into normal group (6 mice) and model group. The model group was intraperitoneally injected with streptozotocin 150 mg / kg, and their tails were cut to measure their random blood glucose after 48 hours. Screen the mice whose blood sugar value exceeds 10.1mmol / L to enter the experiment, and be divided into model group, positive drug group glyburide (3mg / kg.day), pharmaceutical composition FL of the present invention 1 High-dose group (300mg / kg.day gavage), the pharmaceutical composition FL of the present invention 1 Middle dose group (200mg / kg.day gavage), the pharmaceutical composition FL of the present invention 1 The low-dose group (100mg / kg.day gavage) was administered orally orally for four consecutive weeks. After the administration, the serum insulin and fasting blood glucose values of the mice were measured. The experimental data were analyzed by variance analysis, and the results were expressed as X±...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com