Method for preparing etimicin sulfate
A technology of etimicin sulfate and copper acetate, applied in the field of medicine, can solve the problems of cumbersome process, difficult purification, many by-products and the like, and achieves the effects of high yield, high selectivity and few side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
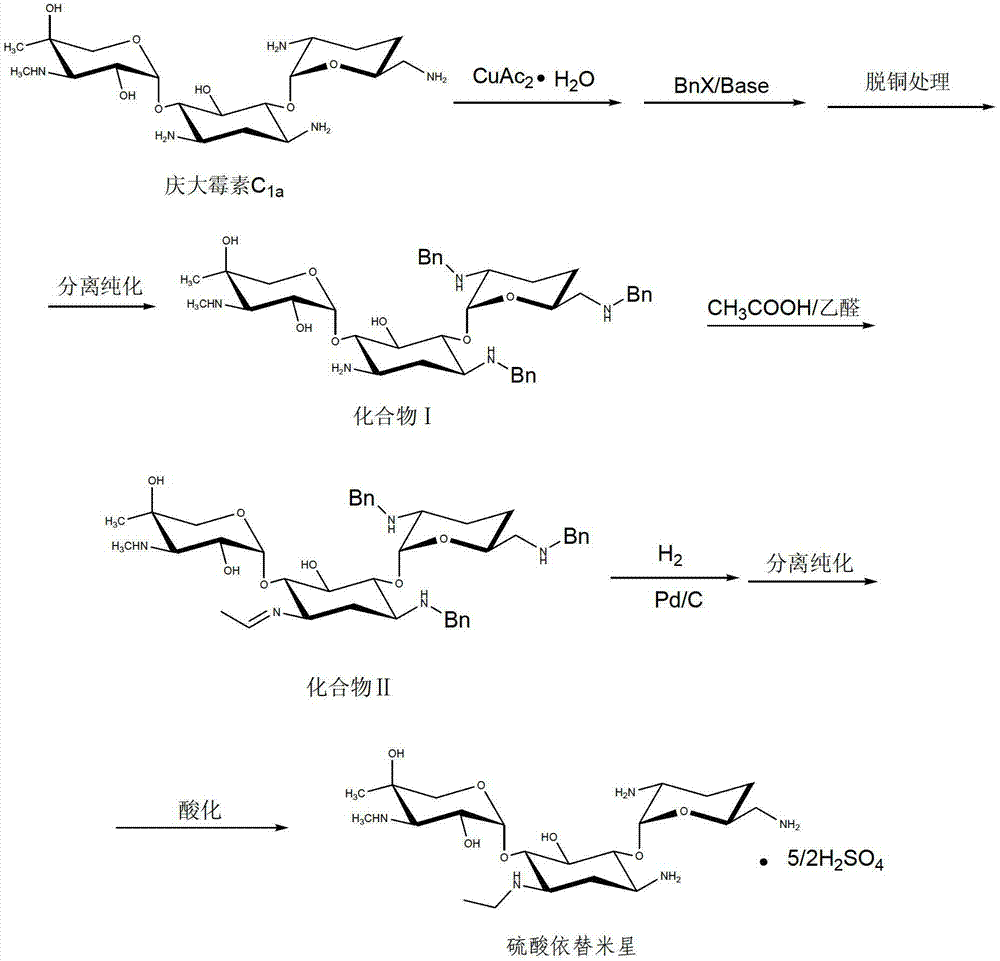
[0021] 15g of gentamicin C with a purity ≥ 98% 1a Dissolve in 150mL DMF, add 16.3g copper acetate monohydrate at room temperature, and react for 20 minutes; then add 17.5g benzyl chloride to the reaction system, raise the temperature to 25°C, and then add 10.2g sodium bicarbonate to the reaction system in batches , 1.5-2 hours to complete the addition, after the addition, the temperature was controlled at 25°C for 4 hours. Pass hydrogen sulfide gas into the obtained reaction solution for decopper treatment, filter to remove insoluble matter, inject the filtrate into a 732 strong acidic cationic resin column for purification, first wash with water until the optical rotation is not greater than 0.050, and then use a concentration of 0.2-2mol / L of ammonia water was analyzed to obtain the analytical solution of the desired product, which was concentrated under reduced pressure to obtain a light yellow oil, which was Compound Ⅰ, EI (m / z): 720 (M+1);
[0022] Disperse compound I i...
Embodiment 2
[0024] 15g of gentamicin C with a purity of not less than 98% 1a Dissolve in 120mL DMSO, add 16.3g copper acetate monohydrate at room temperature, react for 20 minutes, add 23.6g benzyl bromide to the reaction system, raise the temperature to 25°C, then add 12.2g triethylamine in batches to the reaction system, 1.5-2 hours to complete the addition, after the completion of the addition, control the temperature at 25 ° C for 1 hour, pass hydrogen sulfide gas into the obtained reaction liquid for decopper treatment, filter to remove insoluble matter, inject the filtrate into a 732 strong acid cationic resin column for purification, first Rinse with water until the optical rotation is not greater than 0.050, then analyze with ammonia water with a concentration of 0.2-2mol / L to obtain the analytical solution of the desired product, concentrate under reduced pressure to obtain a light yellow oil, which is compound Ⅰ, EI (m / z ): 720 (M+1);
[0025] Disperse compound I in a mixed sol...
Embodiment 3
[0027] 15g of gentamicin C with a purity of not less than 98% 1a Dissolve in 120mL DMSO, add 16.3g copper acetate monohydrate at room temperature, react for 20 minutes, add 17.5g benzyl chloride to the reaction system, raise the temperature to 25°C, then add 10.2g sodium bicarbonate in batches to the reaction system, 1.5-2 hours to complete the addition, after the completion of the addition, control the temperature at 25 ° C for 2 hours, pass hydrogen sulfide gas into the obtained reaction liquid for decopper treatment, filter to remove insoluble matter, inject the filtrate into a 732 strong acid cationic resin column for purification, first Rinse with water until the optical rotation is not greater than 0.050, then analyze with ammonia water with a concentration of 0.2-2mol / L to obtain the analytical solution of the desired product, and concentrate under reduced pressure to obtain a light yellow oil, which is Compound Ⅰ, EI (m / z): 720 (M+1);
[0028] Disperse compound I in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com