Preparation of solid dispersions and oral preparations of paclitaxel and homologous compounds thereof
A technology of solid dispersion and paclitaxel, which is applied in the field of medicine, can solve the problems of clinical application limitations, achieve the effect of tumor suppression, improve oral bioavailability, and improve the effect of bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
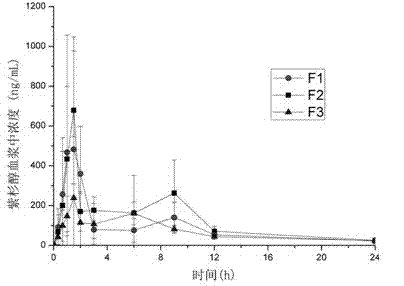
[0034] Example 1: Preparation of Paclitaxel-HPMCAS-LG solid dispersion microspheres (without micronized silica gel)
[0035] Weigh paclitaxel (0.1, 0.3, 0.8g) and hypromellose acetate succinate (HPMCAS-LG) (1.9g, 1.7g, 1.2g) (Japan Shin-Etsu Chemical Co., Ltd.) (General Pharmaceuticals and Polymers) Amount 2g) In a 50mL Erlenmeyer flask with a ground mouth, add 3 ~ 8mL acetone and 3 ~ 6mL dichloromethane, and magnetically stir until completely dissolved. The drug-polymer solution is formed under magnetic stirring. Use distilled water containing 0.05% ~ 0.15% surfactant sodium lauryl sulfonate as a poor solvent, and place 250 mL in a cylindrical preparation container. Under the stirring of a propeller stirring blade (500 ~ 700 rpm), slowly add the above suspension to the poor solvent. Once the suspension is poured into the poor solvent, translucent emulsion droplets are formed. As the dichloromethane and acetone continue to diffuse into the poor solvent, the emulsion droplets gr...
Embodiment 2
[0040] Example 2: Paclitaxel-hypromellose phthalate (HP55) solid dispersion microspheres (without micronized silica gel)
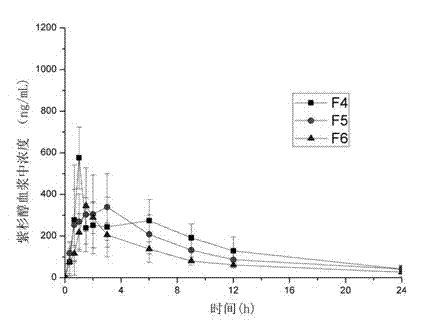
[0041] Weigh paclitaxel (0.1, 0.3, 0.7g) and HP55 (1.9g, 1.7g, 1.3g) (Japan Shin-Etsu Chemical Co., Ltd.) (the total amount of drug and polymer is 2g) into a 50mL Erlenmeyer flask with a grinding mouth. Add 3 ~ 5mL acetone and 6 ~ 8mL dichloromethane, magnetically stir until completely dissolved, and form a drug-polymer solution under magnetic stirring. Other preparation methods are the same as in Example 1. The overall recovery rate is 60 ~ 85%. The obtained preparations are named F4, F5, F6, and the drug content in the prescription is about 5%, 15%, and 35%.
[0042] It can be seen from Table 2 that using hypromellose phthalate (HP55) as a solid dispersion carrier can also achieve better oral bioavailability. As the proportion of drug content in the solid dispersion increases, Tmax decreases, but there is no significant difference between F5 and F6. by f...
Embodiment 3
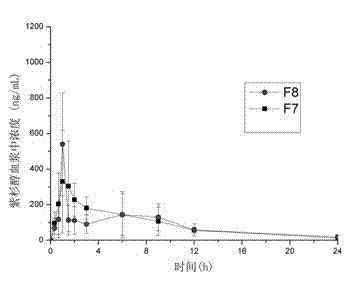
[0046] Example 3: Paclitaxel - Preparation of HPMCAS-LG (or HP55) solid dispersion (without micro-powder silica gel, solvent evaporation method)
[0047] Weigh 100 mg of paclitaxel and mix them with polymer materials HPMCAS-LG (300 mg) and HP55 (300 mg) (the total amount of drugs and polymer in each prescription is 400 mg), and add them to a mixed solvent of 2 mL acetone and 2 mL dichloromethane. After dissolving, spread it evenly on the glass plate. Place in an oven at 45°C for 12 hours to allow the organic solvent to evaporate to dryness. Remove the solid dispersion mixture with a blade, and the obtained preparations are F7 and F8 respectively, and the drug content in the prescription is 25%. It can be seen from Table 3 that the simple solvent evaporation method can also obtain better oral bioavailability. This shows that the combination of drugs and appropriate polymers is one of the key factors to improve the oral bioavailability of drugs.
[0048]
[0049] table Pharmaco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com