Pharmaceutical composition containing granisetron hydrochloride compound
A technology of granisetron hydrochloride and a composition, which is applied to the freeze-dried powder injection preparation of granisetron hydrochloride and the field of preparation thereof, can solve the problems of unsatisfactory dissolution rate, easily fluctuating pH value, insufficient content, etc. Good stability, no irritating effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
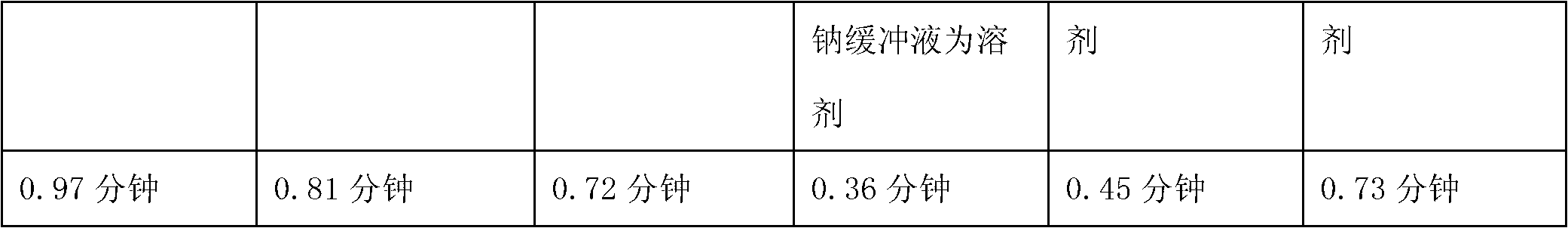
Examples
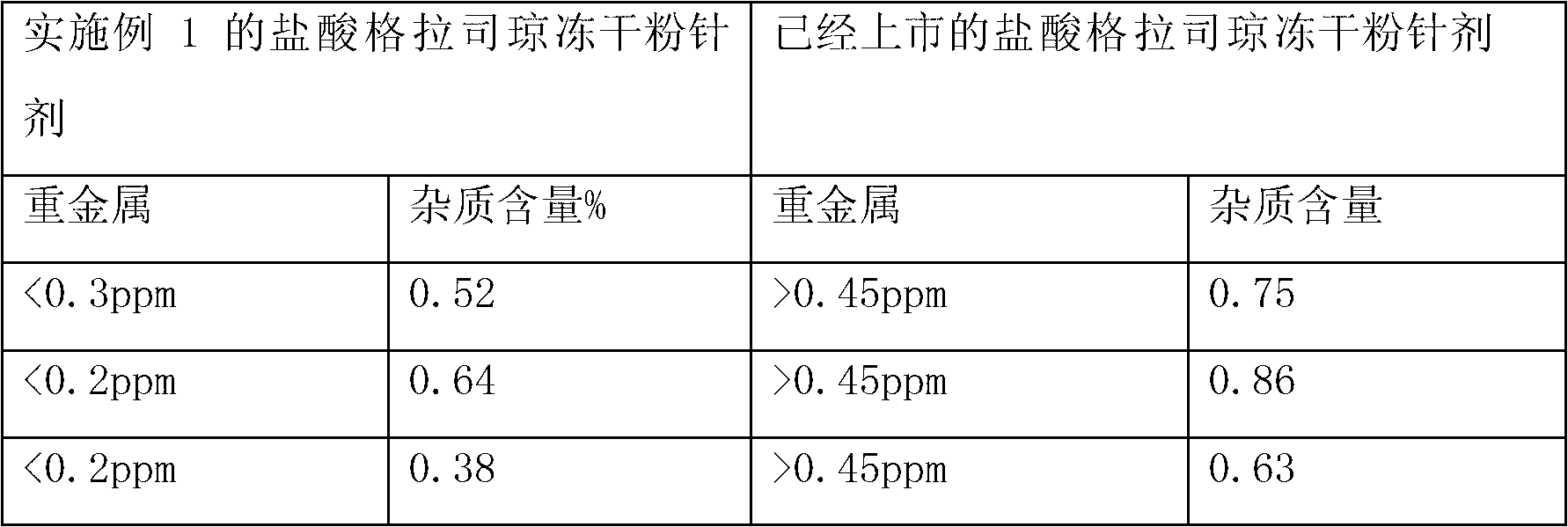
Embodiment 1
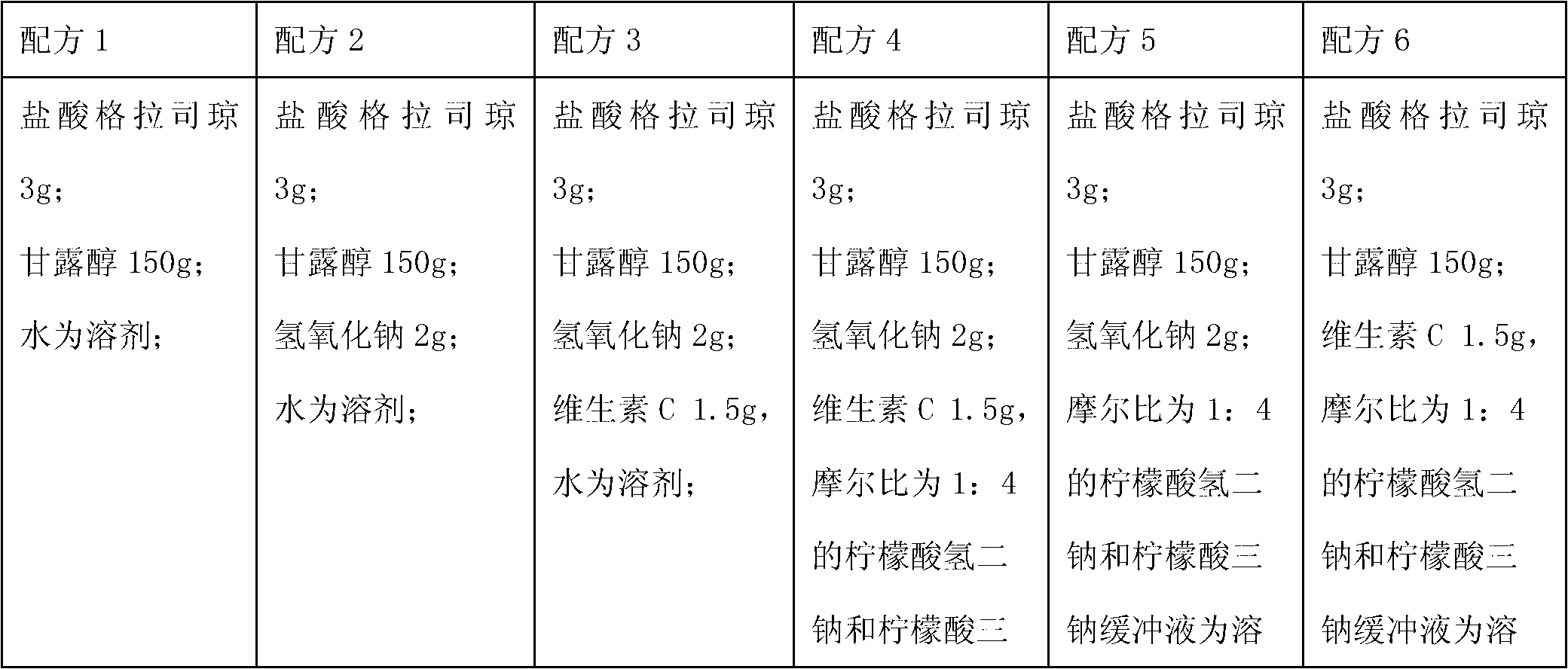
[0054] Granisetron hydrochloride 3g;
[0055] Mannitol 150g;
[0057] Vitamin C 1.5g,
[0058] 2000ml of disodium hydrogen citrate and trisodium citrate buffer solution with a molar ratio of 1:4;
[0059] Preparation
[0060] (1) Weigh the raw and auxiliary materials according to the prescription quantity
[0061] (2) Add mannitol, sodium hydroxide, and vitamin C to 2000ml of disodium hydrogen citrate and trisodium citrate buffer solution with a molar ratio of 1:4, and stir to dissolve.
[0062] (3) Add the prescribed amount of granisetron hydrochloride into the solution and stir to dissolve completely. Stir to combine.
[0063] (4) Measure the pH value of the solution, which is 7.0.
[0064] (5) Fine filter with a 0.2 μm microporous membrane to check the clarity of the solution.
[0065] (6) According to the measurement results, fill the liquid medicine into a vial with a volume of about 2ml.
[0066] (7) Put the sample into a freeze dr...
Embodiment 2
[0069] Granisetron hydrochloride 3g;
[0070] Mannitol 100g;
[0071] Sodium hydroxide 1g;
[0072] Vitamin C 1g,
[0073] 2000ml of disodium hydrogen citrate and trisodium citrate buffer solution with a molar ratio of 1:4;
[0074] Preparation
[0075] Same as Example 1
Embodiment 3
[0077] Granisetron hydrochloride 3g;
[0078] Mannitol 200g;
[0079] Sodium hydroxide 3g;
[0080] Vitamin C 2g,
[0081] 2000ml of disodium hydrogen citrate and trisodium citrate buffer solution with a molar ratio of 1:4;
[0082] Preparation
[0083] With embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com