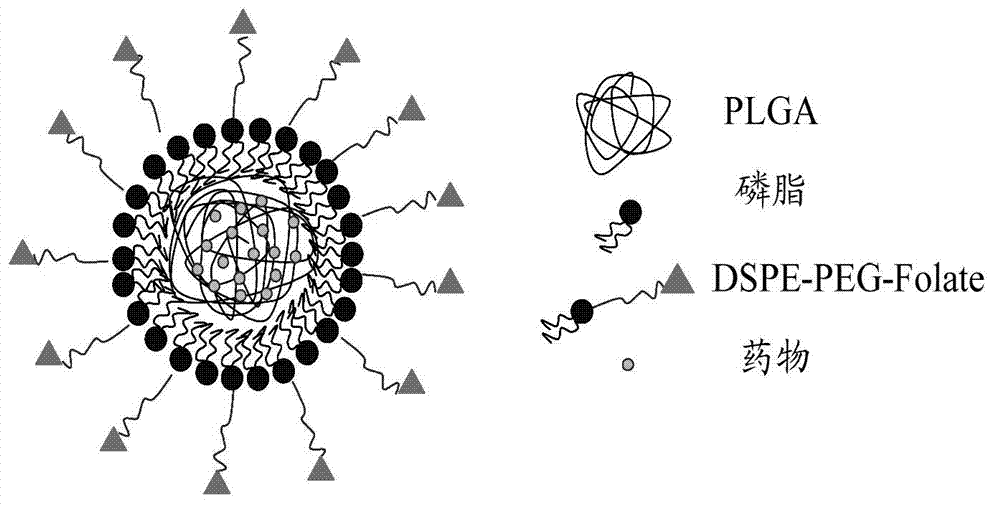
Nano drug delivery system containing polymer and phospholipid and preparation method thereof
A nano-drug-loading and polymer technology, applied in the field of nano-medicine, can solve the problems of low targeting and limited drug loading rate, and achieve controllable biodegradation, simple and easy preparation method, and low toxicity of degradation products. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] In addition, this embodiment also provides a method for preparing a nano drug-loading system containing polymers and phospholipids, including the following steps:
[0021] Step S110, preparing a polylactic acid-glycolic acid copolymer acetonitrile solution with a concentration range of 1-5 mg / mL and a drug methanol solution with a concentration range of 1-5 mg / mL, and mixing the drug methanol solution with the polylactic acid-glycolic acid copolymer acetonitrile solution According to the volume ratio of 5:100 to 20:100, the mixed solution containing the drug and the polylactic acid-glycolic acid copolymer is obtained.
[0022] Step S120, dissolving phospholipids and distearoylphosphatidylethanolamine-polyethylene glycol-folic acid linear polymers in a mixed solution of chloroform and methanol, then adding them to ethanol aqueous solution, and heating to 65°C to obtain phospholipids and linear polymers A mixture of polymers, wherein the concentration of phospholipids is ...
Embodiment 1
[0031] Dissolve an appropriate amount of docetaxel in methanol to obtain a docetaxel solution with a concentration of 2 mg / mL; dissolve an appropriate amount of PLGA in acetonitrile to obtain a PLGA solution with a concentration of 2 mg / mL; take an appropriate amount of soybean lecithin and DSPE-PEG- FoLate was dissolved in a mixture of chloroform and methanol to obtain soybean lecithin at a concentration of 1 mg / mL and DSPE-PEG-FoLate at a concentration of 25 mg / mL, wherein the volume ratio of chloroform to methanol was 9:1. Then 100 μL of docetaxel solution and 1 mL of PLGA acetonitrile solution were mixed ultrasonically to obtain a mixed solution containing docetaxel and PLGA.
[0032]Add 4.8 μL DSPE-PEG-FoLate solution and 180 μL soybean lecithin solution into 3 mL ethanol aqueous solution, heat to 65 °C and stir for 3 minutes to obtain a phospholipid mixture, wherein the mass fraction of ethanol in the ethanol aqueous solution is 4%.
[0033] Add 1.1 mL of the above-menti...
Embodiment 2
[0036] Dissolve an appropriate amount of docetaxel in methanol to obtain a docetaxel solution with a concentration of 2 mg / mL; dissolve an appropriate amount of PLGA in acetonitrile to obtain a PLGA solution with a concentration of 2 mg / mL; take an appropriate amount of soybean lecithin and DSPE-PEG- FoLate was dissolved in a mixture of chloroform and methanol to obtain soybean lecithin at a concentration of 1 mg / mL and DSPE-PEG-FoLate at a concentration of 25 mg / mL, wherein the volume ratio of chloroform to methanol was 9:1. Then take 50 μL of docetaxel solution and 1 mL of PLGA acetonitrile solution and ultrasonically mix to obtain a mixed solution containing docetaxel and PLGA.
[0037] Add 4.8 μL of DSPE-PEG-FoLate solution and 180 μL of soybean lecithin solution into 6 mL of ethanol aqueous solution, heat to 65 °C and stir for 3 minutes to obtain a phospholipid mixture, wherein the mass fraction of ethanol in the ethanol aqueous solution is 4%.
[0038] Add 1.05 mL of the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com