ELISA kit and method for detecting furaltadone metabolites
A technology for furotazone and metabolites, which can be used in the field of enzyme-linked immunoassay to solve problems such as carcinogenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Preparation of antigen, antibody and enzyme-labeled anti-antibody
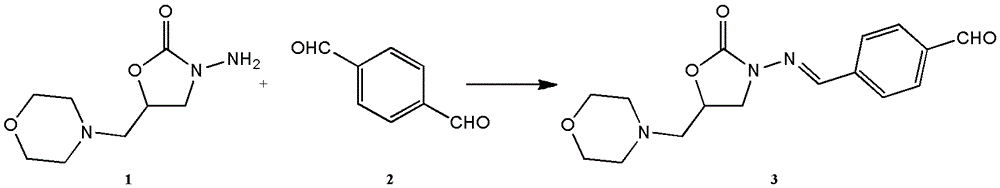
[0042] 1. Preparation of furaltadone metabolite hapten
[0043] A mixture of 2.01g of furaltadone metabolite (AMOZ) and 20ml of DMF was slowly added dropwise at room temperature into 50-100ml of DMF solution of 2.68-5.36g of terephthalaldehyde. The solvent was removed and purified by column chromatography to obtain a pale yellow AMOZ derivative.
[0044] 2. Synthesis of Immunogen
[0045] (1) Dissolve 14 mg of furaltadone metabolite hapten in 1 ml of DMF to obtain solution 1.
[0046] (2) Dissolve 40 mg of BSA in 6 ml of water to obtain solution 2.
[0047] (3) Add solution 1 dropwise to solution 2 to obtain solution 3, and react at room temperature for 24 hours.
[0048] (4) Take NaBH 4 14mg was dissolved in 0.2ml 0.1M NaOH and added to solution 3, and reacted at 4°C for 2h.
[0049] (5) Dialyze with 0.01mol / l PBS at 4°C for 3 days and change the dialysate 3 times a day to remove unrea...
Embodiment 2
[0070] Example 2: The composition of the components of the furaltadone metabolite ELISA kit
[0071] Set up furaltadone metabolite ELISA kit, including the following components:
[0072] (1) A microtiter plate coated with a coating source.
[0073] (2) Enzyme-labeled anti-antibody: horseradish peroxidase-goat anti-mouse anti-antibody.
[0074] (3) Working solution of monoclonal antibody to furaltadone metabolite.
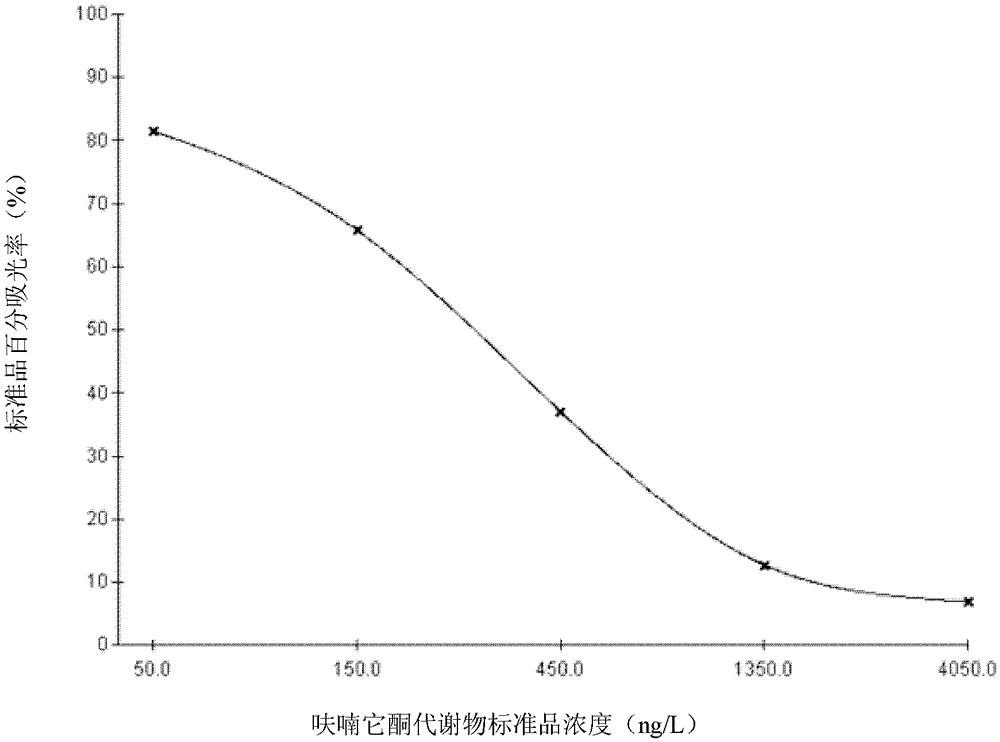
[0075] (4) Standard solution: The standard solution was prepared by gradient dilution method, and 6 bottles of series standard products were obtained, the concentrations were 0 μg / L, 0.05 μg / L, 0.15 μg / L, 0.45 μg / L, 1.35 μg / L, 4.05 μg / L, and high concentration standard 100μg / L, 1mL / bottle.
[0076] (5) The substrate chromogenic solution A is a carbamide peroxide solution, and the substrate chromogenic solution B is a tetramethylbenzidine solution.
[0077] (6) The stop solution is 1-2 mol / L sulfuric acid solution.
[0078] (7) The concentrated washing solution ...
Embodiment 3
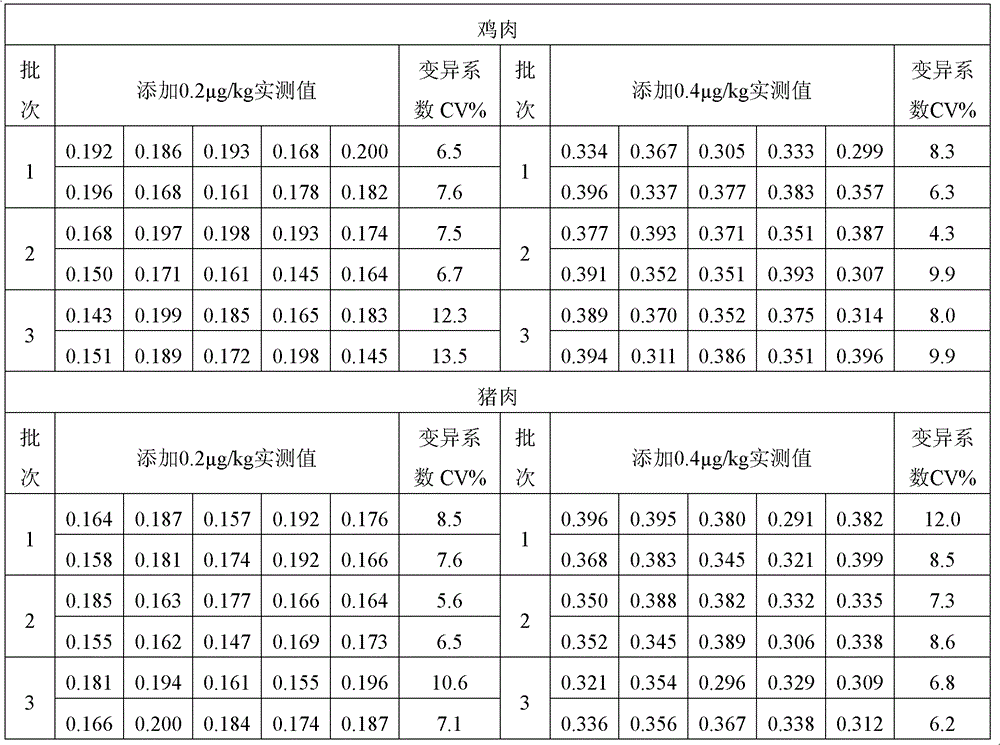
[0081] Example 3: Detection of furaltadone metabolites in samples
[0082] 1. Pretreatment of samples
[0083] (1) Pretreatment method of tissue (muscle, liver and aquatic products) samples
[0084] Weigh 1.0g homogeneous substance, add 4ml deionized water, 0.5ml 1M hydrochloric acid solution (weigh 8.3ml concentrated hydrochloric acid and add deionized water to make up to 100ml) and 100μl derivatization reagent (to the reagent containing 2-nitrobenzaldehyde Add 10ml of methanol to the bottle to dissolve and mix (concentration is 10mM)), shake fully with a shaker for 2min; incubate overnight at 37°C (about 16h); add 5ml of 0.1M dipotassium hydrogen phosphate solution (weigh 22.8g of phosphoric acid trihydrate Dipotassium hydrogen plus 1L deionized water to dissolve and mix), 0.4ml 1M sodium hydroxide solution (weigh 4.0g sodium hydroxide and 100ml deionized water to dissolve and mix), and 5ml ethyl acetate, vibrate vigorously with an oscillator for 30s; 3000g Above, centrifu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com