Recombination expression of HPLC12 type ice structuring protein in bacillus subtilis and preparation method
A technology of Bacillus subtilis and ice-structured proteins, applied in the field of microorganisms, can solve the problems of difficult product separation and purification, low protein activity, low expression level, etc., and achieve the effects of clear understanding of genetic background, simple recovery and purification, and simple secretion of proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] WB800N was purchased from MoBiTec GmbH, Germany, with a product number of PBS022.
[0030] pAL12 was purchased from MoBiTec GmbH, Germany, with a product number of PBS007.
[0031] According to the codon preference of Bacillus subtilis, the gene of HPLC12 ice structure protein was optimized and synthesized by Nanjing GenScript Biotechnology Co., Ltd., and subcloned into pUC57 to obtain pUC57-HPLC12.
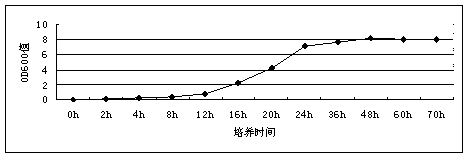
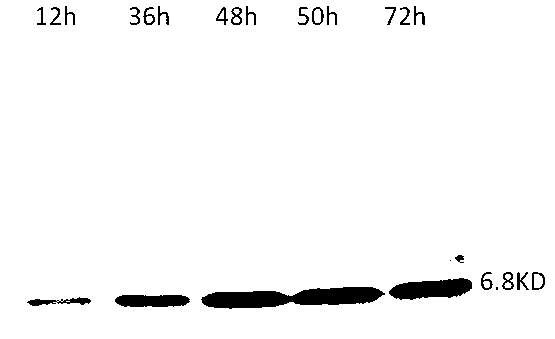
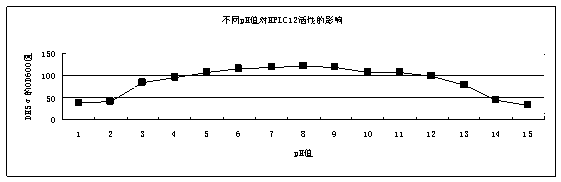
[0032] The preparation method of the HPLC12 type ice structural protein with expression activity provided by the present invention is as follows: the HPLC12 type ice structural protein gene optimized by the gene is connected with the expression vector pAL21 of Bacillus subtilis to obtain a recombinant expression vector, and then the recombinant expression vector is transformed into Introduce the Bacillus subtilis host to obtain a recombinant strain, culture the recombinant strain, and induce at 20° C. for 72 hours to obtain a fermented product with the AFPIII protein.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com