Preparation method of antibacterial agent
An antibacterial drug, tipipenem ester technology, applied in bulk chemical production, organic chemistry and other directions, can solve the problems of low yield, low yield, difficult to achieve, etc., achieves simple process route, simple and easy to obtain raw materials , the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
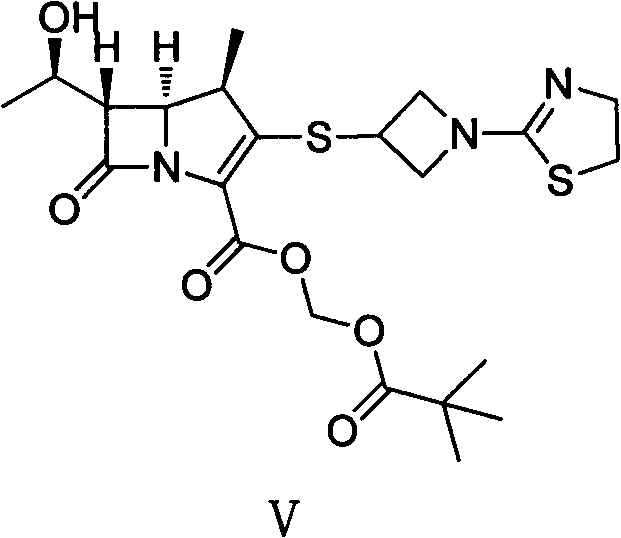
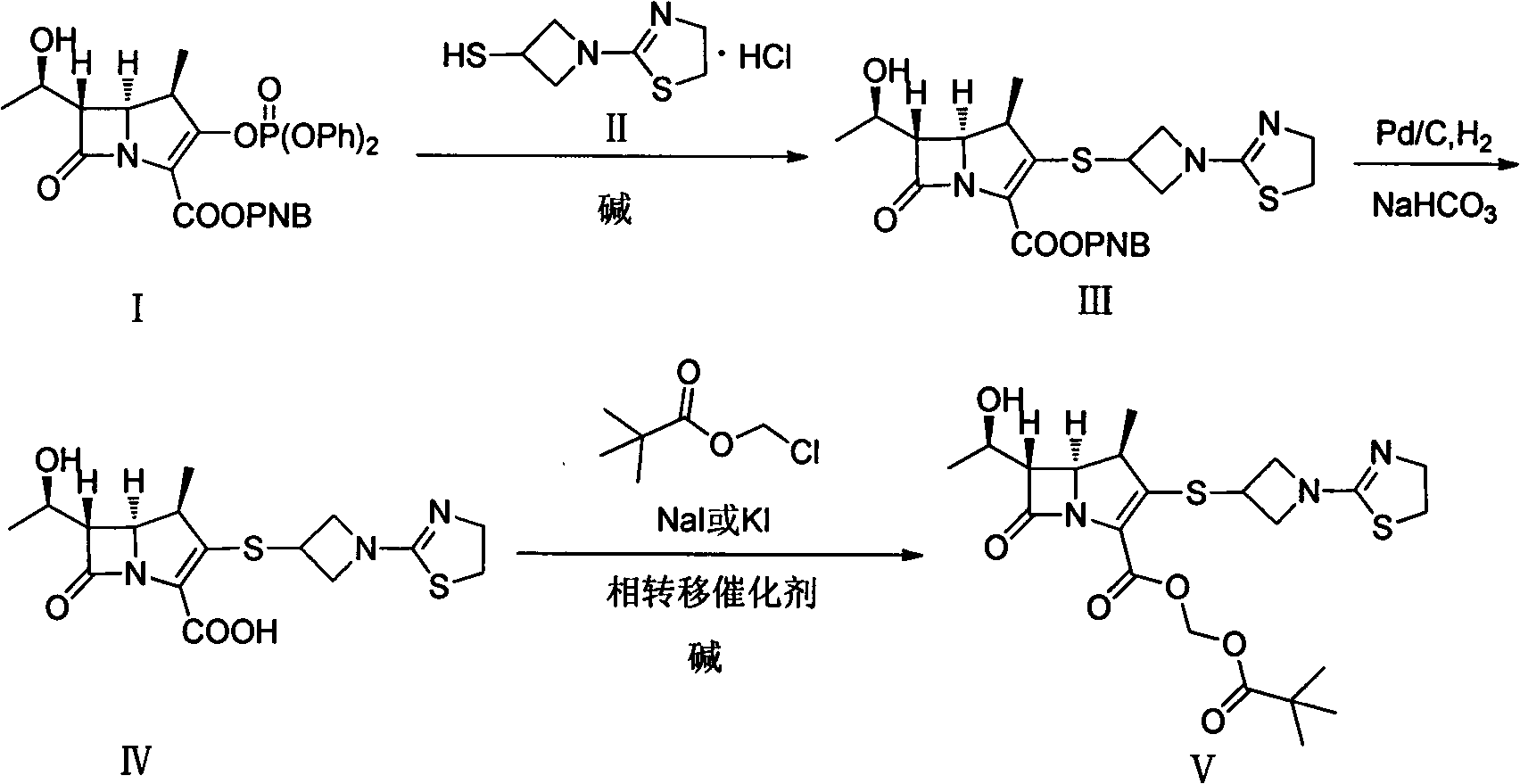
[0028] The first step: (4R, 5S, 6S)-3-((1-(4,5-dihydro-2-thiazolinyl)-3-acridinyl)thio)-6-((R) Preparation of -1-hydroxyethyl)-4-methyl-7-oxo-1-azabicyclo[3.2.0]heptane-2-enyl-2-carboxylic acid p-nitrobenzyl ester
[0029] Add 6-MAP (148.60g), 3-mercaptoazetidine hydrochloride (60.70g) and acetonitrile (1000ml) into a dry three-necked flask, stir at room temperature for 10min, then cool to -20°C, then slowly add DIPEA ( 71.30g), reacted at the same temperature for 10h, added water (600ml) to the reaction system, stirred for 30min after being warmed up to 10°C, filtered, and the filtered solid was washed with acetonitrile / water mixed solution (volume ratio 1:1, 300ml), Washing with isopropanol (300ml) and drying in vacuo afforded (4R,5S,6S)-3-((1-(4,5-dihydro-2-thiazolinyl)-3-acridinyl)thio )-6-((R)-1-hydroxyethyl)-4-methyl-7-oxo-1-azabicyclo[3.2.0]heptane-2-enyl-2-carboxylic acid p-nitro 126.50 g of benzyl ester (III) with a yield of 97% was directly put into the next reacti...
Embodiment 2
[0033] The first step: (4R, 5S, 6S)-3-((1-(4,5-dihydro-2-thiazolinyl)-3-acridinyl)thio)-6-((R) Preparation of -1-hydroxyethyl)-4-methyl-7-oxo-1-azabicyclo[3.2.0]heptane-2-enyl-2-carboxylic acid p-nitrobenzyl ester
[0034] Add 6-MAP (25.00g), 3-mercaptoazetidine hydrochloride (9.80g), DMF (50ml) and ethyl acetate (100ml) into a dry three-necked flask, stir at 0°C for 20min, then slowly add three Ethylamine (10.68g), reacted at 0°C for 6h, warmed up to room temperature, slowly added water (300ml) to the mixture, stirred for 30min, filtered, the solid was washed with ethyl acetate (30ml), and dried in vacuo to obtain (4R, 5S, 6S )-3-((1-(4,5-dihydro-2-thiazolinyl)-3-acridinyl)thio)-6-((R)-1-hydroxyethyl)-4- 28.14 g of p-nitrobenzyl methyl-7-oxo-1-azabicyclo[3.2.0]heptane-2-enyl-2-carboxylate, with a yield of 67%, was directly put into the next reaction.
Embodiment 3
[0036] The second step: (4R, 5S, 6S)-3-((1-(4,5-dihydro-2-thiazolinyl)-3-acridinyl)thio)-6-((R) Preparation of -1-hydroxyethyl)-4-methyl-7-oxo-1-azabicyclo[3.2.0]heptane-2-enyl-2-carboxylic acid
[0037] Add (4R, 5S, 6S)-3-((1-(4,5-dihydro-2-thiazolinyl)-3-acridinyl)thio)-6- ((R)-1-hydroxyethyl)-4-methyl-7-oxo-1-azabicyclo[3.2.0]heptane-2-enyl-2-carboxylic acid p-nitrobenzyl ester ( III) (9.33g), NaHCO 3 (0.76g), 10% Pd / C catalyst (4.00g), n-butanol (100ml) and water (125ml), under 400KPa ~ 500KPa hydrogen pressure, 20 ℃ reaction 7h, filter catalyst, filtrate with 1.0mol / L dilute hydrochloric acid solution to adjust the pH value to 5.6, separate the liquids, concentrate the water phase under reduced pressure to about 40ml, slowly add it to acetone (800ml) cooled in an ice-water bath, stir at the same temperature for 3h, and filter to obtain (4R, 5S, 6S)-3-((1-(4,5-dihydro-2-thiazolinyl)-3-acridinyl)thio)-6-((R)-1-hydroxyethyl)-4 -Methyl-7-oxo-1-azabicyclo[3.2.0]heptane-2-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com