Medicine composition with carbazole alkaloid in clausena plants as antineoplastic activity ingredient and preparation method and application thereof
A technology for carbazole alkaloids and compositions, which is applied in the preparation of antitumor drugs, the field of tumor cell growth inhibitors, can solve the problems of easy adsorption of samples, insufficient sensitivity, easy loss of samples, etc., and achieves controllability and reproducibility. The effect of good performance, less sample loss and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
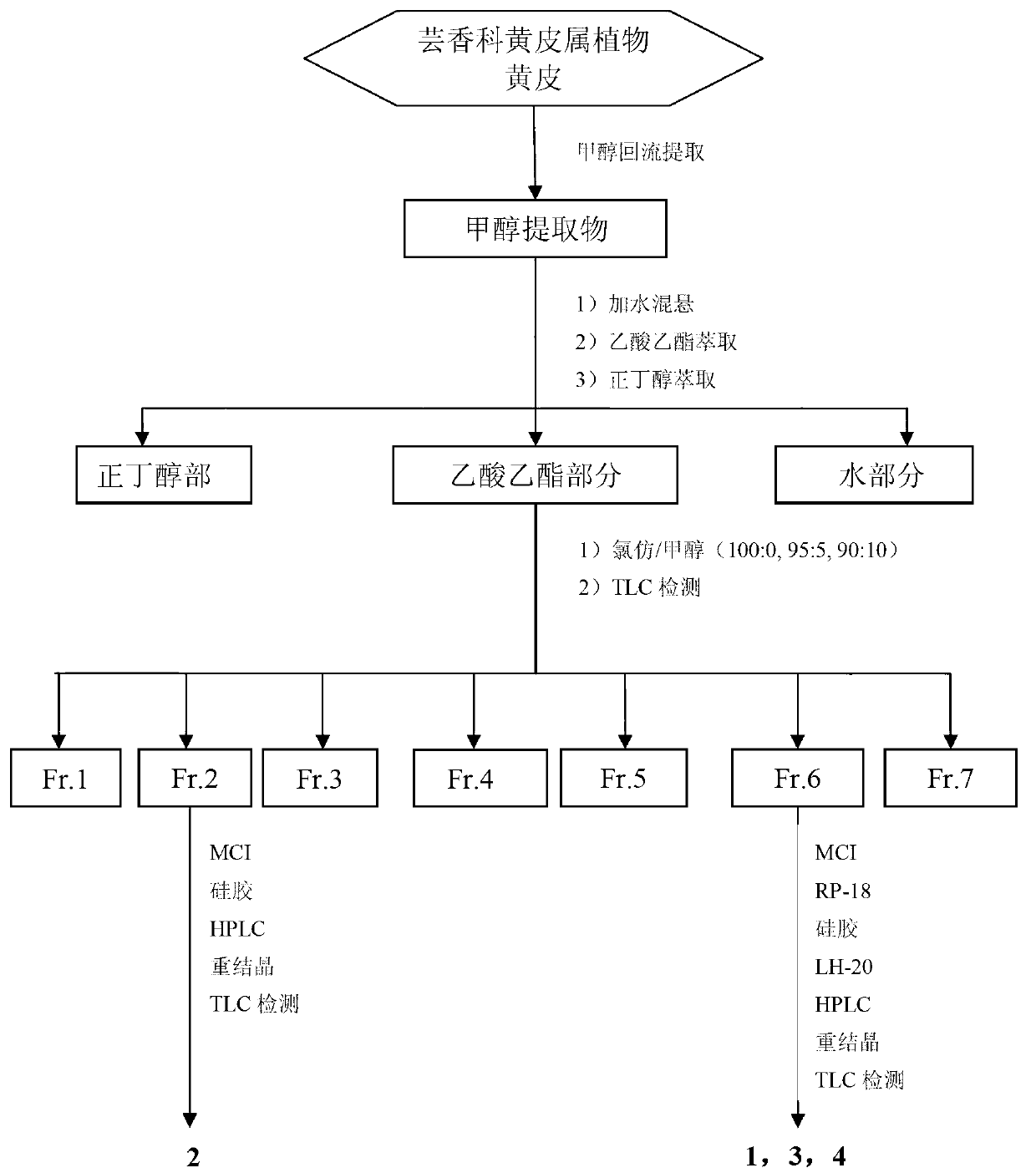
[0033] Preparation and structural identification of carbazole alkaloids clausine D (1), 6-methoxyheptaphylline (2), mafaicheenamine C (3) and claulansine G (4) from plants of the genus Pampas:
[0034]Take 27 kg of rhizomes of Wampucum japonica, after drying and crushing, reflux extraction with methanol for 3 times (100 L × 3 times) for 4 h, and concentrate the extract under reduced pressure to obtain 800 g of methanol extract; After adding water to suspend, extract fully with ethyl acetate and n-butanol successively, and extract three times with equal volumes (30 L × 3 times), recover the solvent to obtain 406 g of ethyl acetate, 158 g of n-butanol and 236 g of water . The ethyl acetate part was subjected to silica gel column chromatography, eluted with 100:0, 95:5, 90:10 chloroform / methanol gradient, thin layer chromatography, combined with TLC detection method, according to the different polarity of carbazole alkaloids Combined into seven components Fr.1 - Fr.7; each of th...
Embodiment 2
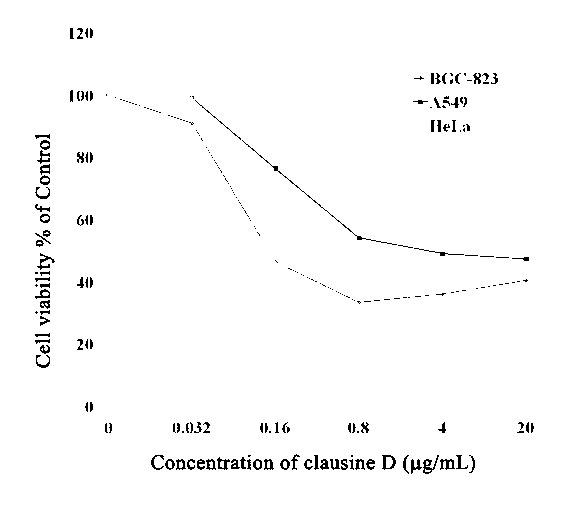
[0039] Cytotoxicity test results of clausine D (1), 6-methoxyheptaphylline (2), mafaicheenamine C (3) and claulansine G (4) of the present invention on tumor cells BGC-823, HeLa and A549. The experimental principles, methods and results are as follows:
[0040] Experimental principle: After the co-culture of drugs and cells, the survival rate of tumor cells is detected. Sulforhodamine B (SRB) is a water-soluble protein dye. The sulfonate anion in its molecule binds to the basic amino acid of intracellular protein in a weakly acidic environment, and dissolves the intracellular substance with alkaline solution. SRB and measure its absorbance value, the protein content in the cell can be known from the content of SRB, and this represents the cell survival rate.
[0041] Experimental method: (1) Cell inoculation: The above three tumor cells were prepared into a single cell suspension with a culture medium containing 10% fetal bovine serum, and 5×10 cells were used for each well. ...
Embodiment 3
[0047] Compound 1-4 obtained in Example 1 was added with 4% sulfuric acid ethanol solution, pH = 4, filtered, and dried to prepare sulfate compound 1-4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com