Chemiluminescent enzyme-linked immunoassay detection kit for staphylococcal enterotoxin C
A staphylococcal intestinal and chemiluminescent enzyme technology, applied in the field of immunoassay, can solve the problems of poor detection sensitivity, many interference factors, low sensitivity, etc., and achieve the effects of strong specificity, good repeatability and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Preparation of Staphylococcus aureus Enterotoxin C Monoclonal Antibody and Screening of Coating Antibody and Detection Antibody Pairing
[0046] (1) Preparation of anti-Staphylococcus aureus enterotoxin C monoclonal antibody: The purified natural Staphylococcus aureus enterotoxin C antigen standard was provided by the Institute of Microbiology and Epidemiology, Academy of Military Medical Sciences. Eight-week-old BALB / c mice were used as immunized animals, with Staphylococcus aureus enterotoxin C as the immunogen, the first immunization dose was 50 μg / mL, and the immunogen was dissolved in normal saline and an equal volume of Freund’s complete adjuvant Emulsifier, multi-point subcutaneous injection. The second immunization was carried out after an interval of 3 weeks. The dose and route were the same as the first immunization, and the adjuvant was Freund's incomplete adjuvant. After 2-3 weeks, the third immunization was carried out, the dose was the same as ...
Embodiment 2
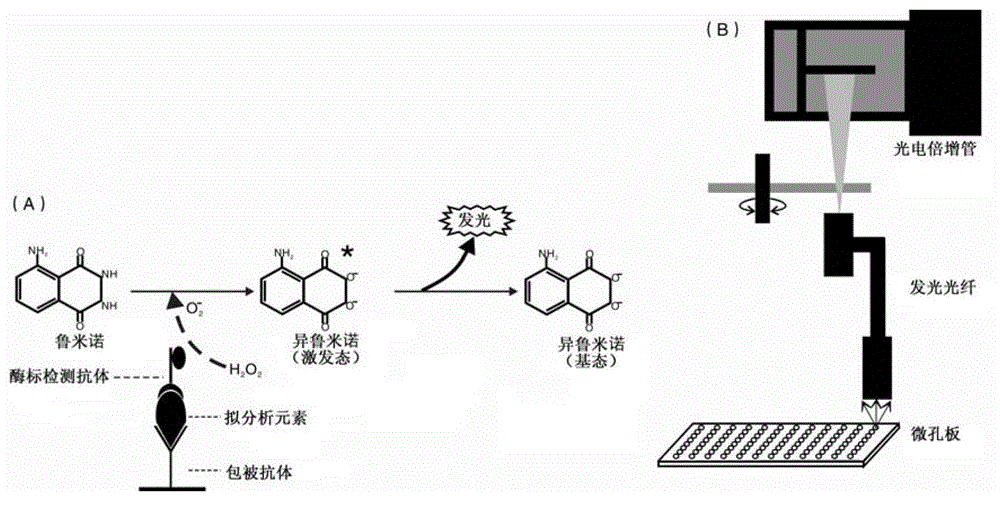
[0053] Example 2: Establishment of Chemiluminescent ELISA Method
[0054] (1) Selection of optimal coating antibody concentration:
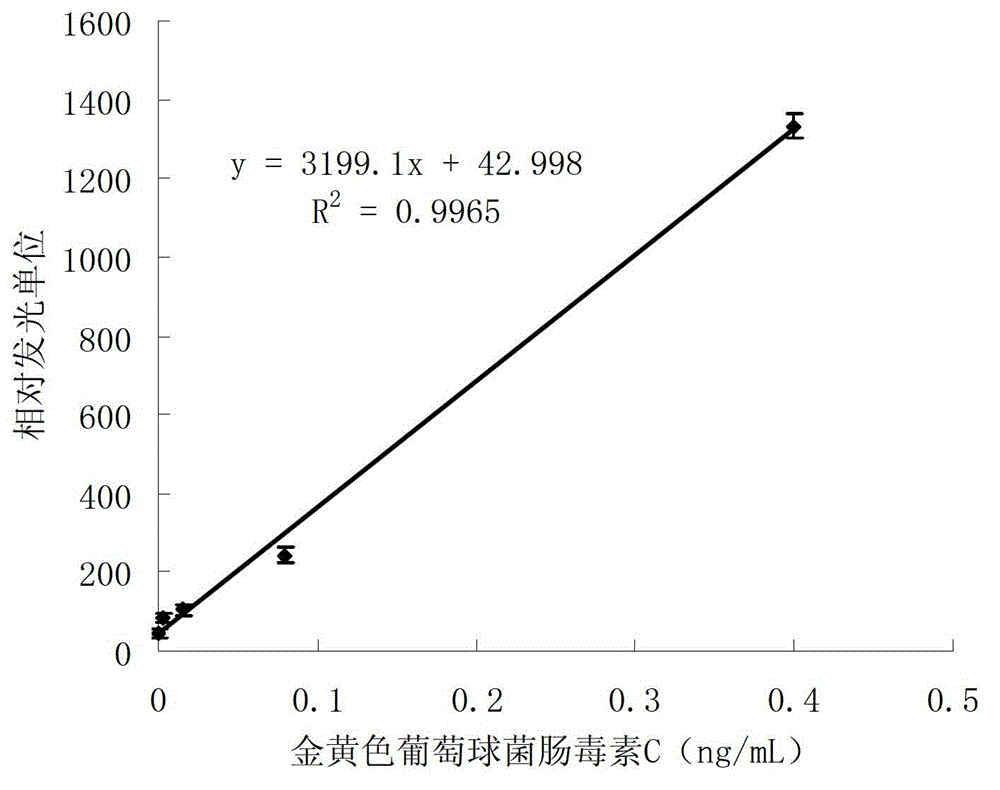
[0055] The coating antibody was coated on the chemiluminescent enzyme-linked plate at serial dilutions of 1.25 μg / mL, 2.5 μg / mL, 5 μg / mL, 10 μg / mL, 20 μg / mL and 40 μg / mL, 100 μL / well, incubated overnight at 4 °C, Wash the plate 3 times with washing solution; block with 250 μL / well blocking solution, place at room temperature for 1 hour, and wash the plate 3 times; add 0.01 ng / mL Staphylococcus aureus enterotoxin C standard antigen, 100 μL / well, and each coating The antibody concentration was used as blank control wells, incubated at 37°C for 1 hour, and washed the plate 3 times; respectively added 1:16000 diluted anti-Staphylococcus aureus enterotoxin C detection antibody, incubated at 37°C for 1 hour, washed the plate 5 times; added 100 μL / well of chemiluminescence solution, and measure the luminescence value. According to the ratio of the me...
Embodiment 3
[0065] Example 3: Application of chemiluminescent ELISA detection kit for detection of staphylococcal enterotoxin C in different matrices
[0066] This example is used to evaluate the accuracy and practicability of the staphylococcal enterotoxin C chemiluminescence ELISA detection kit of the present invention in detecting staphylococcus aureus enterotoxin C in different matrices.
[0067]The standard substance of Staphylococcus aureus enterotoxin C was diluted to different concentrations with environmental matrix river water, food matrix milk, body fluid matrix human serum or human urine, and the content of Staphylococcus aureus enterotoxin C was detected by chemiluminescence enzyme immunoassay, and calculated The ratio of the measured concentration to the actual concentration added is the recovery rate.
[0068] (1) Pre-coat the chemiluminescence enzyme-linked plate with the coating antibody: dilute the coating antibody to 2.5 μg / mL with the coating solution and add it to the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com