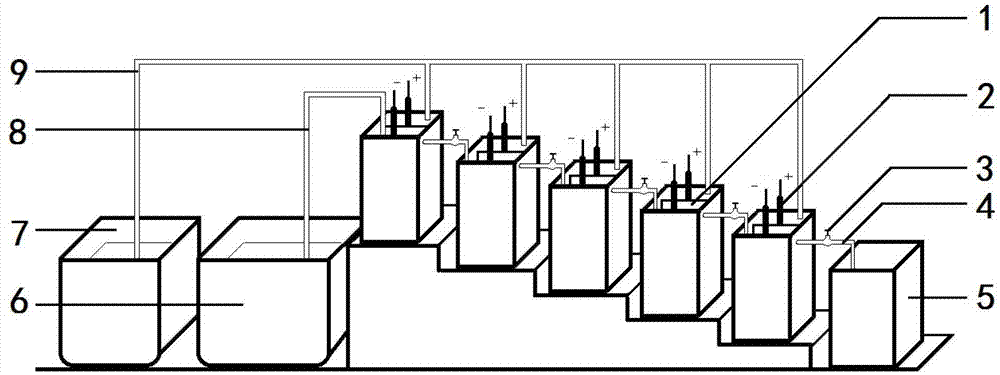
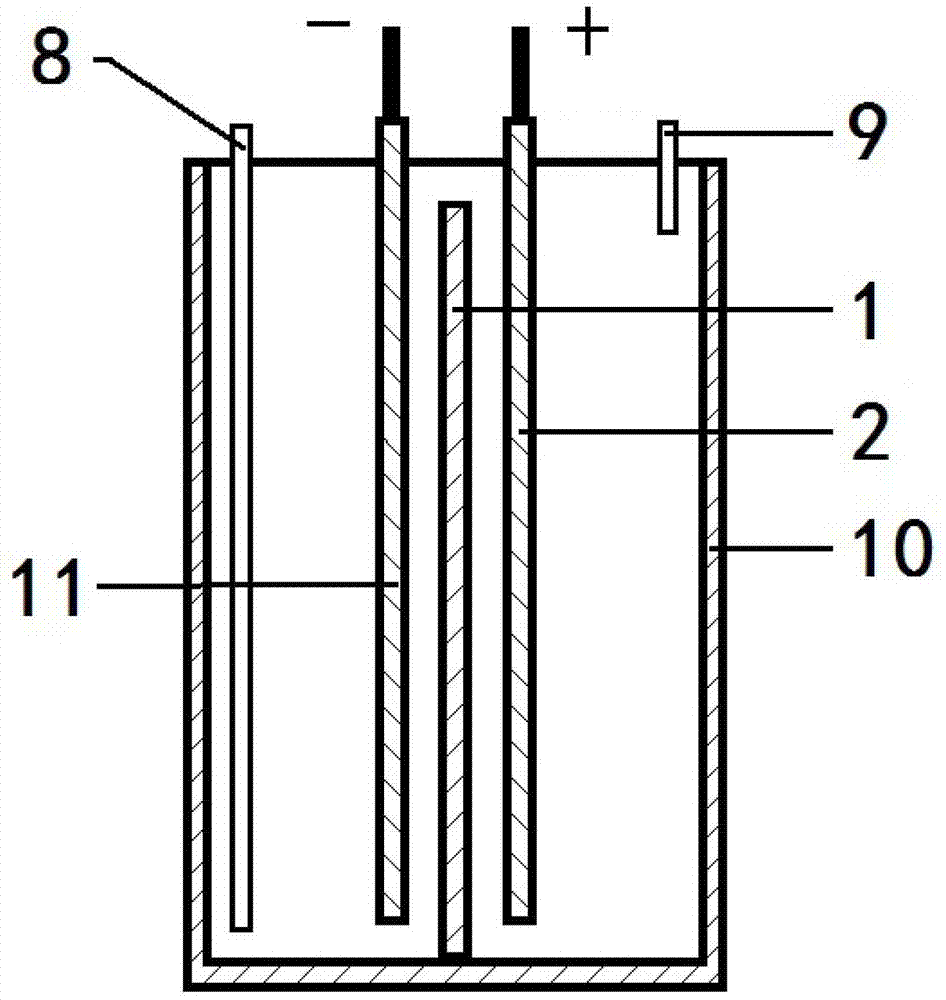
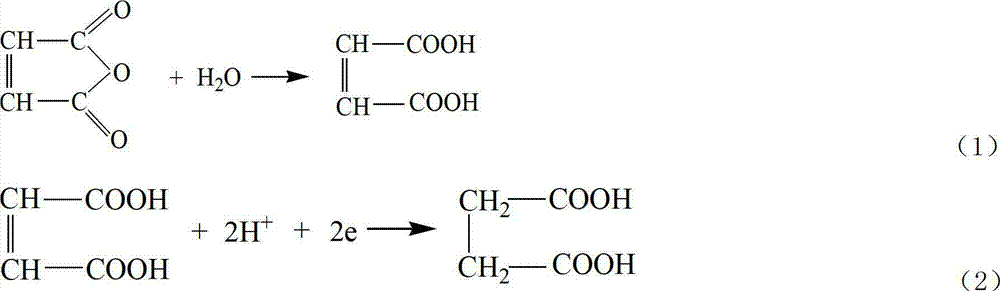
Method for producing butanedioic acid based on serial overflowing method of bipolar membrane electrolytic cell
A technology of electrolytic cells and bipolar membranes, applied in electrolytic organic production, electrolytic processes, electrolytic components, etc., can solve problems such as heavy workload, unfavorable for safe production of rectifiers, troublesome operation, etc., to reduce IR drop, pole distance Effects of small, mild preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Preparation of bipolar membranes.
[0042] Dissolve 5g of SBS in 40mL of toluene and dioxane mixed solution (V / V=3 / 1), add 10-60% freshly distilled acrylic acid (AA) and 1.0% BPO (benzoyl peroxide, with AA mass count). In a nitrogen atmosphere, react at a constant temperature of 80° C. for 3 hours, and then stop the reaction by cooling down to obtain the SBS-g-PAA cation exchange membrane layer emulsion.
[0043]5g of SBS was dissolved in 20mL of toluene and 20mL of dioxane mixed solution, and 3g of N,N-dimethylaminoethyl acrylate (DMAEMA) and 1.0% BPO (based on the mass of DMAEMA) were added. In a nitrogen atmosphere, react at a constant temperature of 80°C for 3h. The SBS-g-DMAEMA graft copolymer containing tertiary amine groups is obtained, which is the SBS-g-DMAEMA anion exchange membrane layer emulsion.
[0044] Cast the SBS-g-PAA cation exchange membrane layer emulsion on flat glass, and then cover it with SBS-g-DMAEMA anion exchange membrane layer emulsion whe...
Embodiment 2
[0054] The preparation of the bipolar membrane is the same as in Example 1.
[0055] Preparation of succinic acid
[0056] Weigh 11g of maleic anhydride and dissolve it in 100mL of water, adjust the pH to 1 with 1mol of sulfuric acid, and add it to the electrolytic cell after it dissolves. Electrolyte temperature 35°C, current density 100mA cm -2 , the total current is 2A, and ten bipolar membrane monomer electrolyzers are connected in series. The last collection tank collects the electrolyte to obtain the product succinic acid after distillation. During the electrolysis process, the electrolyte raw material tank in the cathodic chamber continuously supplies electrolyte to the cathodic chamber of the uppermost bipolar membrane monomer electrolyzer, and the electrolyte water supplement tank in the anode chamber continuously replenishes water to each anode chamber.
[0057] The apparent yield of succinic acid is 112%, the apparent current efficiency is 105%; the average elect...
Embodiment 3
[0059] The preparation of the bipolar membrane is the same as in Example 1.
[0060] Preparation of succinic acid
[0061] Weigh 12g of maleic anhydride, dissolve it in 100mL of water, adjust to pH=1 with 1mol of sulfuric acid, and add it to the electrolytic cell after it dissolves. Electrolyte temperature 45°C, current density 25mA cm -2 , the total current is 2A, and ten bipolar membrane monomer electrolyzers are connected in series. The last collection tank collects the electrolyte to obtain the product succinic acid after distillation. During the electrolysis process, the electrolyte raw material tank in the cathodic chamber continuously supplies electrolyte to the cathodic chamber of the uppermost bipolar membrane monomer electrolyzer, and the electrolyte water supplement tank in the anode chamber continuously replenishes water to each anode chamber.
[0062] The apparent yield of succinic acid is 113%, the apparent current efficiency is 113%, and the average electroly...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com