Method for extracting and separating rare earth elements in sulfuric acid system
A separation method and technology for rare earth elements, applied in the field of extraction, can solve the problems of difficult stripping of medium and heavy rare earths, high residual acid in stripping liquid, large consumption of acid and alkali, and achieve good interface phenomenon, low extraction acidity, and acid consumption. less effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Potassium hydroxide (8.4g, 0.15mol) was dissolved in 42ml ethanol and added dropwise to [C 25 h 54 N][Cl] (40g, 0.1mol), stirred at room temperature for 1 hour, filtered off the precipitated potassium chloride, and obtained clear [C 25 h 54 N][OH] ionic liquid.
[0045] To the prepared [C 25 h 54 Add 2-ethylhexyl mono-2-ethylhexylphosphonic acid (P507) (0.08mol) to N][OH] (0.1mol), stir at room temperature for 2 hours, take the upper ionic liquid organic phase, and wash with deionized water for 3 time, and then reclaim the solvent ethanol by distillation under reduced pressure, and the product methyl trioctyl ammonium 2-ethylhexyl mono-2-ethylhexylphosphonic acid ionic liquid [A336][P507].
Embodiment 2
[0047] Effect of solution equilibrium pH on distribution ratio of single rare earth ions in [A336][P507] extraction.
[0048] 1.0mL0.05mol / L [A336][P507]n-heptane solution and 4.0mL containing Na 2 SO 4 Mix with an aqueous solution of La(III), where Na in the aqueous solution 2 SO 4 Concentration is 1.0mol / L, La(III) concentration is 7.5×10 -4 mol / L, shake at a constant temperature of 25°C for 0.5h, and measure the equilibrium pH value of the water phase after shaking and the corresponding concentration of La(III) in the water phase. Calculate the extraction partition ratio. When the solution equilibrium pH value is different, the extraction distribution ratio will change.
Embodiment 3~16
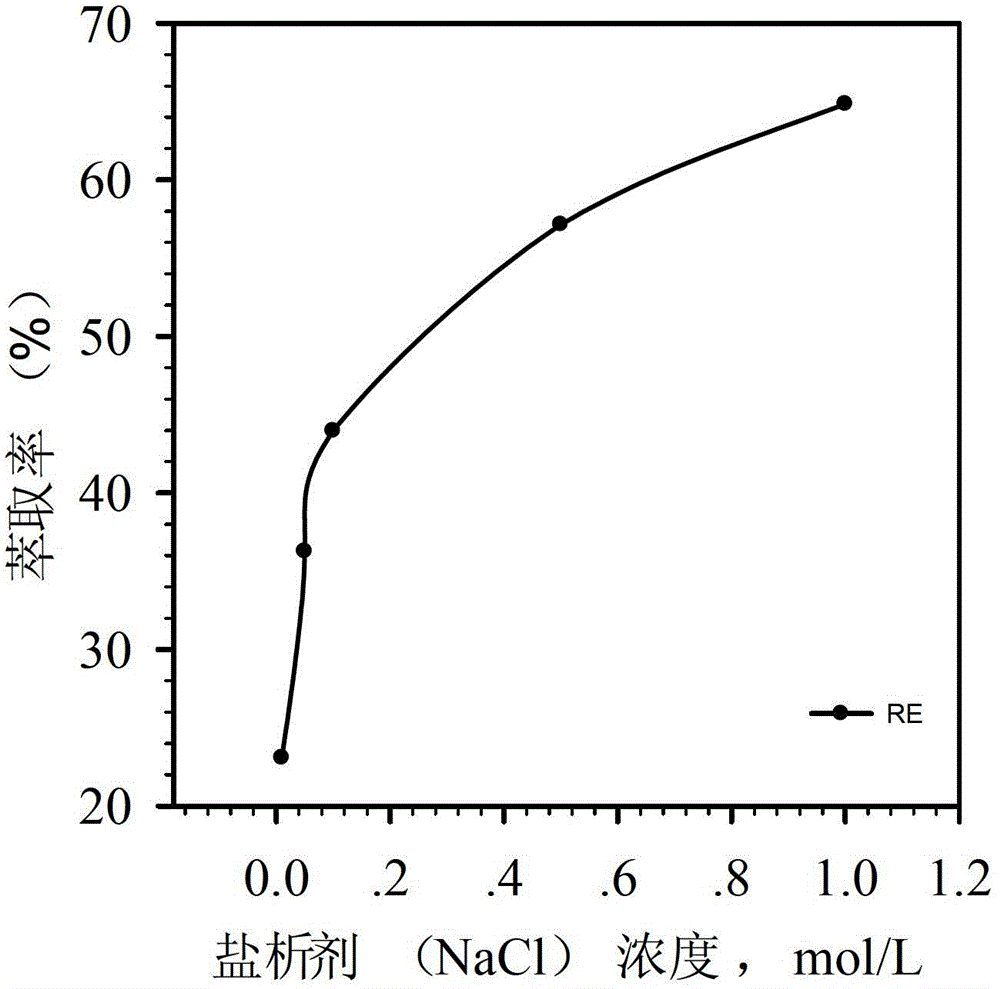
[0050] Using the same extraction method as in Example 2, Ce(III), Pr(III), Nd(III), Sm(III), Eu(III), Gd(III), Tb(III), Dy(III) were obtained respectively ), Ho(III), Er(III), Tm(III), Yb(III), Lu(III) and Y(III) extraction distribution ratio, that is, the distribution ratio of different rare earth ions at different pH values. figure 1 When [A336][P507] is used as the extractant, the dot plot of the equilibrium pH value of the solution and the extraction distribution ratio of rare earth ions can be seen from the figure, when the equilibrium pH value increases, the distribution ratio of rare earth ions increases. It is obtained by measuring the equilibrium pH value, and the equilibrium pH value is in the range of 1 to 7, and various rare earth ions can complete the extraction process, and the extraction acidity of the extraction agent is relatively low. The calculated average separation coefficient of rare earth elements is 1.36.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com