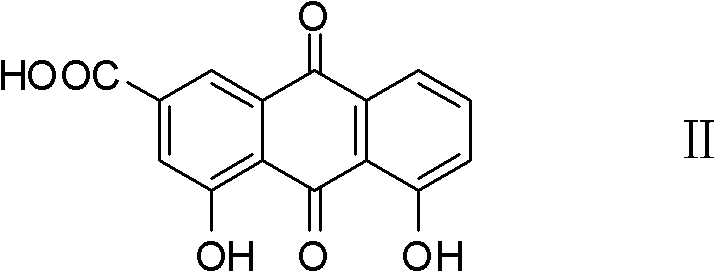
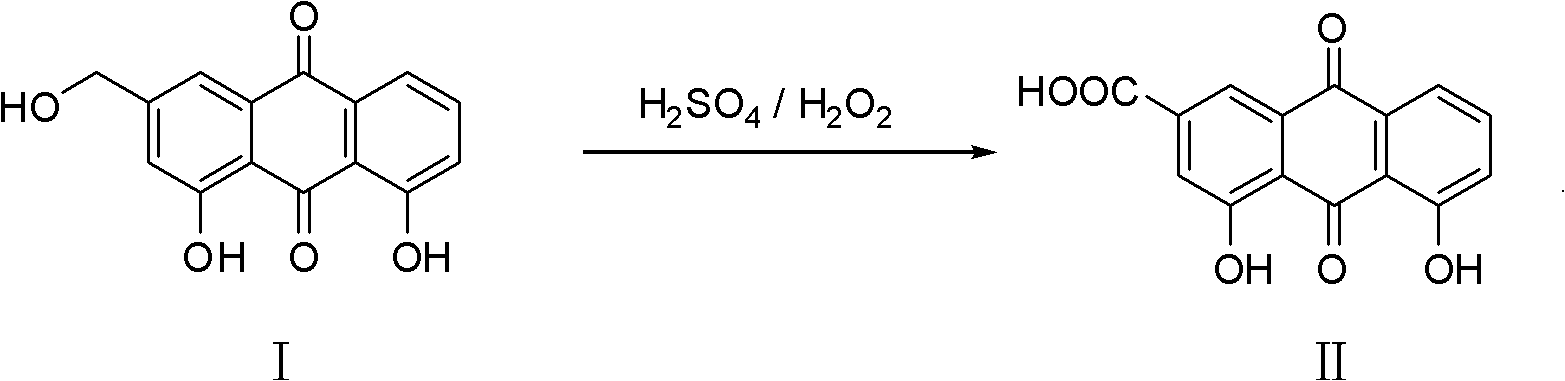Novel synthesis method for rhein
A synthetic method and technology of rhein, applied in the field of compounds, can solve the problems of low yield, environmental pollution, and great harm to human body, and achieve the effects of high yield, high purity, and reduced environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The more specific synthetic method of rhein of the present invention comprises the following process:
[0023] Add 50.0 grams of 30% hydrogen peroxide into a clean 250mL four-necked bottle, cool to -5.0~5.0°C, then slowly add 12.0 grams of 98% concentrated sulfuric acid dropwise at a controlled temperature of -5.0~5.0°C, and stir for 2.0 ~2.5 hours, then slowly add 7.0 grams of aloe-emodin under control of the temperature at 15.0-20.0°C, and keep the temperature at 15.0-20.0°C for 2.0-4.0 hours after the addition.
[0024] Add 300.0 g of 5% DMF aqueous solution into another 500 ml four-necked bottle, and cool to 15.0-20.0°C.
[0025] After the heat preservation is completed, slowly add the reaction feed liquid at a controlled temperature of 20.0-35.0°C to the DMF aqueous solution in a 500ml four-necked bottle, and keep stirring at 20.0-35.0°C for 2.0-2.5 hours after the addition.
[0026] After the heat preservation is completed, filter, wash with 100.0 g of drinking w...
Embodiment 1
[0029] Add 50.0 grams of 30% hydrogen peroxide into a 250ml four-necked bottle, cool to -5.0°C, then slowly add 15.0 grams of 98% concentrated sulfuric acid dropwise at temperature control -5.0°C--3.0°C, and stir for 2.5 hours after the dropwise addition. Slowly add 7.0 grams of aloe-emodin at a temperature of 15.5-17.5°C, and keep the temperature at 15.5-17.5°C for 2.5 hours to react. At the end of the heat preservation, slowly add the reaction solution into a 500ml four-necked bottle containing 300.0 grams of 5.0% DMF aqueous solution, control the temperature of the addition process at 25.0-30.0°C, and keep stirring for 2.5 hours after the addition. Suction filtration, washing with 100.0 grams of drinking water, drying to obtain 6.72 grams of rhein, HPLC content 98.6%, yield 91.3%
Embodiment 2
[0031] Add 50.0 grams of 30% hydrogen peroxide into a 250ml four-neck bottle, cool to 0.0°C, then slowly add 7.0 grams of 98% concentrated sulfuric acid dropwise at a temperature of 0.0-3.0°C, stir for 2.5 hours after the addition, and control the temperature at 16.5-18.5 Slowly add 7.0 grams of aloe-emodin at ℃, and keep the temperature at 16.5-18.5 to react for 3.0 hours after the addition. At the end of the heat preservation, slowly add the reaction solution into a 500ml four-necked bottle containing 300.0 grams of 5.0% DMF aqueous solution, control the temperature of the addition process at 35.0° C., and stir for 2.0 hours after the addition is complete. Suction filtration, washing with water, drying to obtain 6.42 grams of rhein, HPLC content 98.8%, yield 87.2%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com